All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
2023 ELN recommendations Part 1: diagnosis, prognosis, and assessment of adult ALL
The initial management of adults with acute lymphoblastic leukemia (ALL) is an unmet medical need identified by the European Working Group of the European LeukemiaNet (ELN). The ALL Hub is pleased to present a summary of the first part of the 2023 ELN recommendations, published by Gokbuget et al.1 in Blood, in which we focus on the diagnosis, prognosis, and assessment of adult ALL.
Key recommendations1
Diagnostic approaches
- The diagnostic work-up of ALL relies on a combination of morphological, immunophenotypic, and genetic/cytogenetic-based assessments (Figure 1).
- A bone marrow aspiration is routinely done to confirm diagnosis; for patients with a dry aspirate, a bone marrow biopsy is recommended.
- Further genomics, such as gene expression profiling, copy number alteration, genome-wide, and next-generation sequencing can be used to better define subgroups.
- Next-generation sequencing has enabled the identification of novel mutations, including RAS, JAK/STAT pathway of T and B-cell lineage, and NOTCH1/FBXW7 lesions in T-cell ALL (T-ALL).
- Copy number alterations have enabled the identification of mutations affecting the cell cycle (CDKN2A/B and RB1) and lymphoid development (IKZF1, PAX5, and VPREB1).
Figure 1. Diagnostic work-up of ALL*
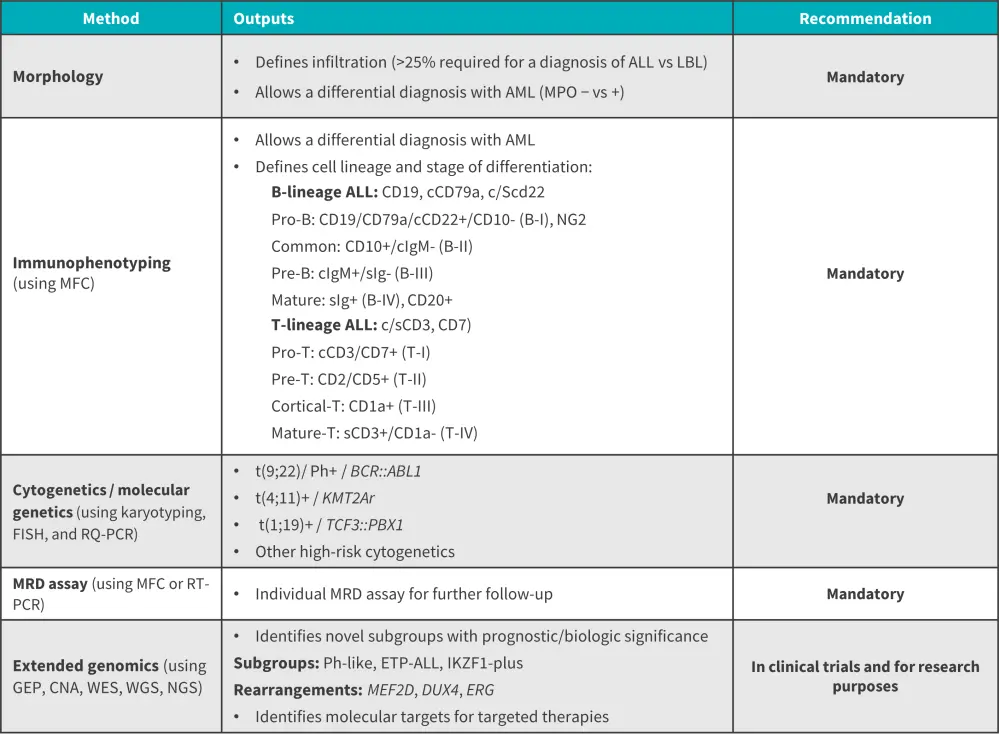
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNA, copy number alteration; ETP-ALL, early T-cell precursor ALL; GEP, gene expression profiling; LBL, lymphoblastic leukemia; MFC, multicolor flow cytometry; MPO, myeloperoxidase; MRD, minimal residual disease; NGS, next-generation sequencing; Ph-like, Philadelphia chromosome-like; RQ-PCR, real-time quantitative polymerase chain reaction; WES/WGS: whole exome/whole genome sequencing.
*Data from Gokbuget, et al.1
Prognostic factors and risk stratification
- Different risk subgroups can be identified based on MRD response and prognostic factors
- Standard risk: no poor prognostic factors and/or favorable post-induction MRD
- High risk: poor prognostic factors and/or poor MRD response
- There are several patient and disease-related risk factors in adult ALL (Figure 2).
Figure 2. Potential prognostic and predictive factors in adult ALL*
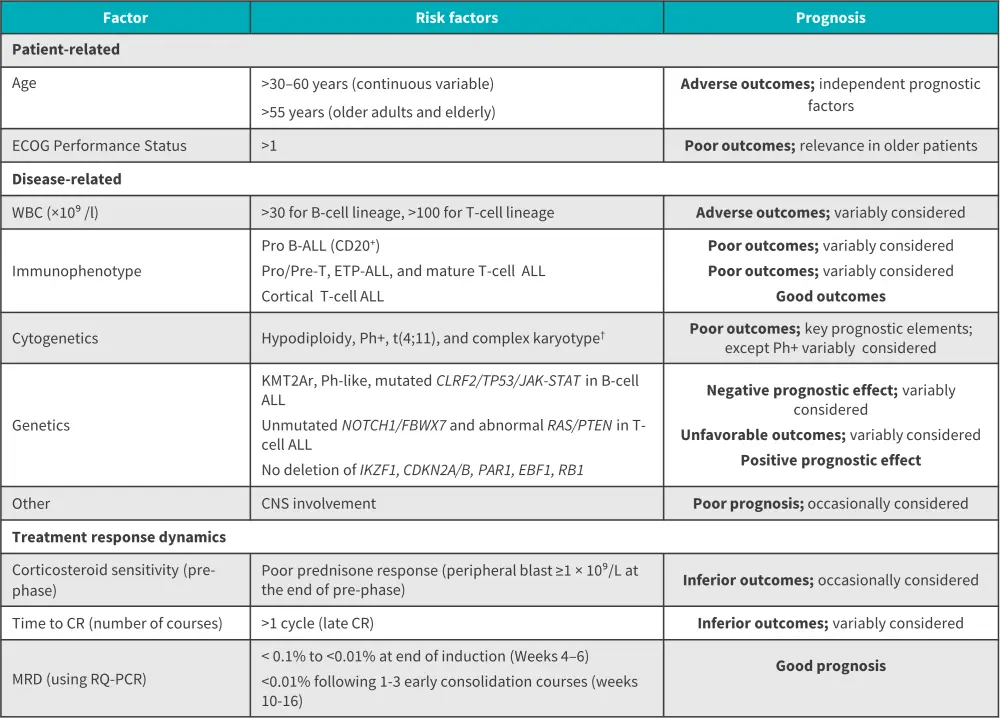
ALL, acute lymphoblastic leukemia; CNS, central nervous system CR, complete remission; ECOG, Eastern Cooperative Oncology Group; ETP, early thymic precursor; ETP-ALL, early T-cell precursor ALL; MRD, minimal residual disease; Ph+, Philadelphia chromosome-positive; Ph-like, Philadelphia chromosome-like; RQ-PCR, real-time quantitative polymerase chain reaction; WBC, white blood cell count.
*Data from Gokbuget, et al.1
†Definition of complex karyotype: ≥5 chromosomal abnormalities excluding patients with an established translocation.
Response criteria and definitions1
- Criteria for bone marrow response assessment in ALL are based on acute myeloid leukemia (Figure 3) and criteria for extramedullary response are based on non-Hodgkin lymphoma.
- Positron emission tomography analysis should be considered for patients with persistent extramedullary involvement after induction and early consolidation
- In clinical trials, positron emission tomography negative status can be considered as complete remission for patients with complete remission with incomplete hematologic recovery and partial remission.
Figure 3. Response criteria for ALL*
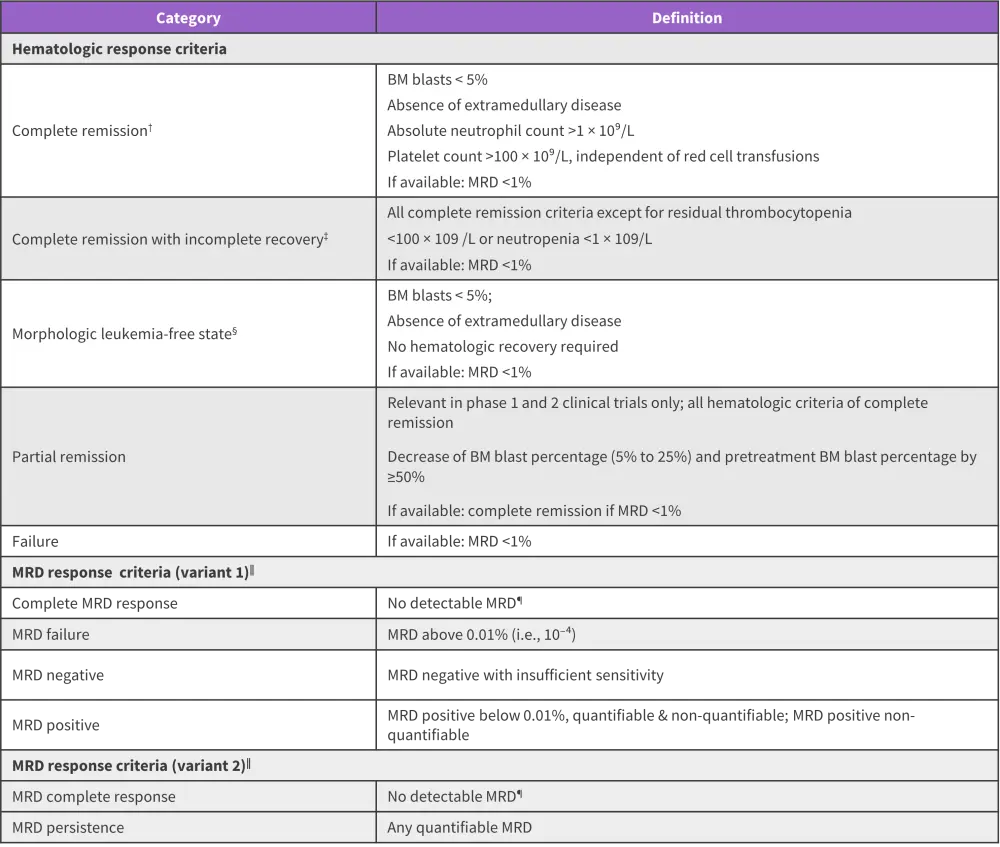
ALL, acute lymphoblastic leukemia; BM, bone marrow; MRD, minimal residual disease
*Data from Gokbuget, et al.1
†All criteria must be fulfilled.
‡complete remission with incomplete hematologic recovery is of value in protocols using intensified induction or double induction strategies
§This category may be useful in the clinical development of novel agents within phase I clinical trials, in which a transient morphologic leukemia-free state may be achieved at the time of early response assessment.
‖Confirmation of any MRD response requires the application of standardized methods with minimum technical requirements in reference laboratories.
¶Confirmation of negative MRD requires technical requirements for the establishment of the sensitivity of each time-point to be fulfilled.
A summary of the guidelines for diagnosis, classification, and response assessment in patients with ALL is summarized in Figure 4.
Figure 4. Recommendations for diagnosis, classification, and response assessment in adult ALL*
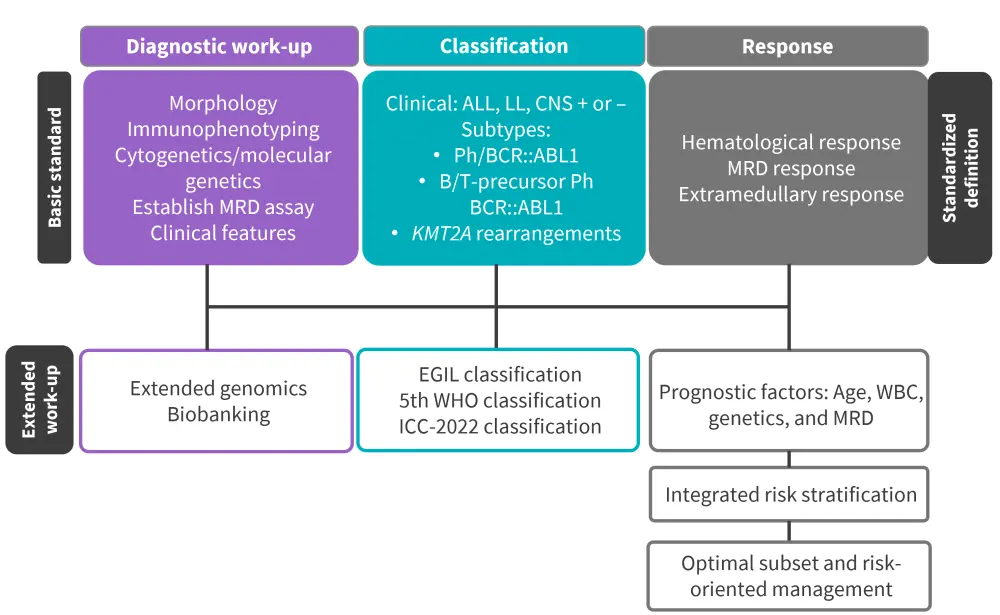
ALL, acute lymphoblastic leukemia; CNS, central nervous system; EGIL, European Group for the Immunological Characterization of Leukemias; ICC, International Consensus Classification; LL, lymphoblastic lymphoma; MRD, minimal residual disease; Ph, Philadelphia-chromosome; WBC, white blood cell count; WHO, World Health Organization.
*Data from Gokbuget, et al.1
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

