All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
AALL1931: Erwinia asparaginase in ALL
At the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Maese1 presented new data on behalf of the Children’s Oncology Group on the AALL1931 study (NCT04145531), which investigated a recombinant Erwinia chrysanthemi asparaginase, JZP458, in patients with acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LBL). Similar to other asparaginases used in the treatment of ALL and LBL, JZP458 induces apoptosis in cancer cells.2 A unique benefit of JZP458 is that it can be used in patients who are hypersensitive to E.coli products.1 JZP458 was approved by the FDA in 2021 at a dose of 25 mg/m2 for the treatment of ALL and LBL in patients ≥1 month old.1,3
Study design1
- Part A of this ongoing phase II/III trial enrolled 167 patients with ALL or LBL who experienced Grade ≥3 allergic reactions to E.coli-derived asparaginase. The study was split into two parts:
- Part A involved administering JZP458 intramuscularly.
- Part B involved administering JZP458 intravenously.
- Both part A and part B involved patients receiving six doses of JZP458 on a Monday–Wednesday–Friday (MWF) schedule.
- Maese presented results from part A of the study. Results from part B of the study are expected later this year.
- Part A consisted of three patient cohorts:
- Cohort 1a received JZP458 at a dose of 25 mg/m2 (MWF).
- Cohort 1b received JZP458 at a dose of 37.5 mg/m2 (MWF).
- Cohort 1c received differential dosing of 25 mg/m2 on Monday and Wednesday, and 50 mg/m2 on Friday.
- The safety follow-up was at 30 days.
- The primary endpoints included safety and tolerability of the drug, with efficacy measured by the proportion of patients achieving a nadir serum asparaginase activity assessment (NSAA) level of ≥0.1 IU/mL in the last 72 hours of the first course of JZP458. A population pharmacokinetics (PopPK) model was developed based on SAA data to allow better dosing decisions and simulate efficacy in a larger population (2,000 patients).
Results1
A total of 167 patients were dosed in part A of this trial; patient characteristics are shown below in Table 1.
Table 1. Patient characteristics*
|
B-ALL, B-cell acute lymphoblastic leukemia; B-LBL, B-cell lymphoblastic lymphoma; T-ALL, T-cell acute lymphoblastic leukemia; T-LBL, T-cell lymphoblastic lymphoma. |
|||
|
Characteristics |
Cohort 1a |
Cohort 1b |
Cohort 1c |
|---|---|---|---|
|
Median age, years (range) |
11 |
8 |
12 |
|
<6, n (%) |
9 (27) |
24 (29) |
11 (22) |
|
6 to <12, n (%) |
9 (27) |
34 (41) |
14 (27) |
|
12 to <18, n (%) |
7 (21) |
20 (24) |
18 (35) |
|
≥18, n (%) |
8 (24) |
5 (6) |
8 (16) |
|
Male, n (%) |
17 (52) |
55 (66) |
31 (61) |
|
Primary disease, n (%) |
|
|
|
|
B-ALL |
27 (82) |
60 (72) |
37 (73) |
|
T-ALL |
4 (12) |
13 (16) |
9 (18) |
|
B-LBL |
0 |
0 |
1 (2) |
|
T-LBL |
2 (6) |
10 (12) |
4 (8) |
|
Median time (range) since last asparaginase dose received, days |
9 |
10 |
10 |
|
Eligibility criteria met, n (%) |
|
|
|
|
Grade ≥3 allergic reaction to an E.coli-derived asparaginase |
27 (82) |
75 (90) |
44 (86) |
|
Silent inactivation |
3 (9) |
3 (4) |
1 (2) |
|
Allergic reaction with inactivation |
3 (9) |
5 (6) |
6 (12) |
Efficacy outcomes
- The proportion of patients reaching the primary endpoint of an NSAA level of ≥0.1 IU/mL in the last 72 hours (first treatment course) is presented in Figure 1.
- Mean SAA levels (first treatment course) in the last 72 hours were
- 0.16 IU/mL for cohort 1a;
- 0.33 IU/mL for cohort 1b; and
- 0.47 IU/mL for cohort 1c.
Figure 1. Patients achieving an NSAA level of ≥0.1 IU/mL in the last 72 hours*
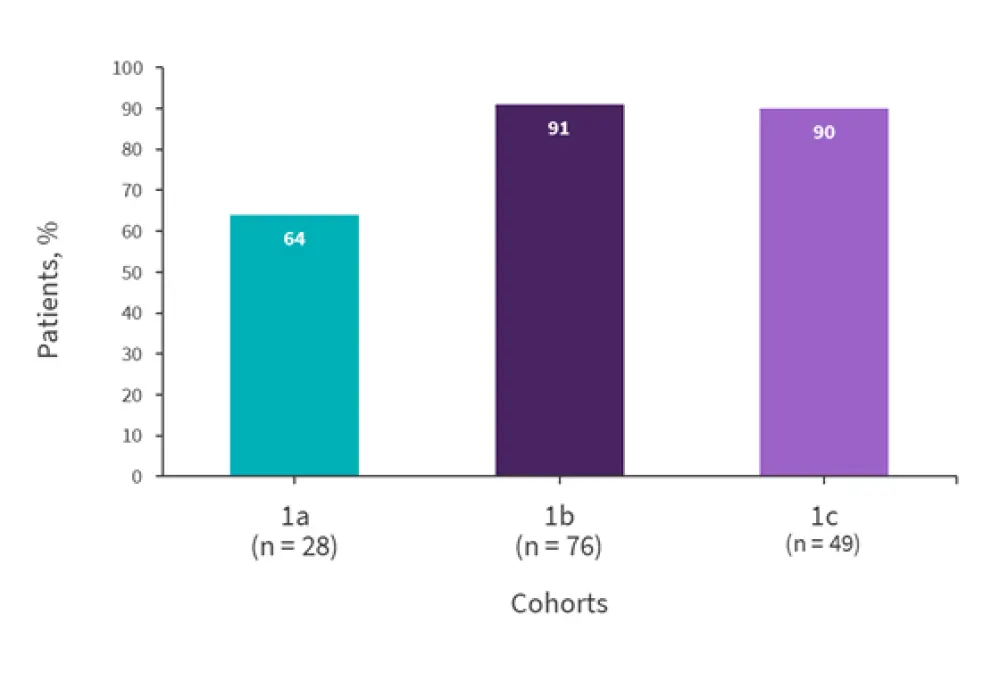
NSAA, nadir serum asparaginase activity.
*Adapted from Maese.1
- The model simulated that 92% of patients in Cohort 1c would reach ≥0.1 IU/mL in the last 72 hours of the first treatment course; the observed proportion was 90%.
- Following intramuscular administration, the PopPK model that best described the PK of JZP458 was a 1-compartment intramuscular model with mixed-order absorption and linear elimination.
- The geometric mean half-life for JZP458 was estimated at 19.1 hours following intramuscular administration.
Safety analysis
- Overall, 6%, 17%, and 10% of treatment-related adverse events lead to drug discontinuation in cohorts 1a, 1b, and 1c, respectively.
- The primary reasons for discontinuation included pancreatitis, drug hypersensitivity, and anaphylactic reaction.
- There were no treatment-related adverse events leading to death.
Conclusion
The findings from part A of the AALL1931 trial demonstrate the efficacy of intramuscular JZP458 at 25/25/50 mg/m2 in patients with ALL or LBL. The safety profile shows the treatment is well tolerated, with a similar safety profile to other asparaginases. Data from part B of this study, expected later this year, will provide more clarification on the potential of using JZP458 intravenously, and the best route of administration of the drug.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

