All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Five-year follow-up of tisagenlecleucel in pediatric and young adult patients with R/R B-ALL: ELIANA trial
Introduction
Relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (ALL) remains a challenge to treat in pediatric and young adult populations, historically characterized by poor prognosis and increasing morbidity. Tisagenlecleucel, an autologous CD-19 directed chimeric antigen receptor (CAR) T-cell therapy, showed significant efficacy and a tolerable safety profile in heavily pretreated adults with R/R B-ALL in a primary analysis of the ELIANA phase II trial (NCT02435849).1 Recently, 3-year results of the ELIANA trial were published in the Journal of Clinical Oncology by Laetsch, et al.2 During the European Hematology Association (EHA) 2022 Congress, Susana Rives presented the final results from a 5-year follow-up of patients treated in phase II of the ELIANA trial.3 Below, we summarize the key findings.
Study design
ELIANA is an ongoing, phase II, open-label, multicenter global study in patients aged 3–21 years with R/R B-ALL and bone marrow blasts ≥5%. Patients with prior CD19-directed or gene therapy and isolated extramedullary disease relapse were excluded. Patients ≤50 kg received infusion of tisagenlecleucel at 0.2–5.0 × 106 CAR+ viable T cells/kg and 0.1–2.5 × 108 CAR+ viable T cells for patients >50 kg. Lymphodepletion therapy prior to infusion included fludarabine 30 mg/m2 (IV daily for four doses) and cyclophosphamide 500 mg/m2 (IV daily for two doses).
The primary endpoint was overall remission rate, defined as the complete remission (CR) rate + complete remission with incomplete hematologic recovery (CRi). Secondary endpoints included minimal residual disease status, relapse-free survival (RFS), duration of response, overall survival (OS), event-free survival (EFS), cellular kinetics, and safety.
Baseline characteristics
A total of 79 patients were infused with tisagenlecleucel with a median age of 11 years, 57% were male. All patients were heavily pretreated with a median of three prior lines of therapy and 61% had prior allogeneic hematopoietic stem cell transplantation (AlloSCT). All patients had a high leukemic burden at induction (Table 1).
Table 1. Baseline and clinical characteristics*
|
AlloSCT, allogeneic hematopoietic stem cell transplantation; CNS, central nervous system. |
|
|
Characteristic, % (unless otherwise stated) |
All patients (N = 79) |
|---|---|
|
Median age (range), years |
11 (3–24) |
|
Male |
57 |
|
Prior AlloSCT |
61 |
|
Median prior lines of therapy (range), n |
3 (1–8) |
|
Disease status |
|
|
Primary refractory |
8 |
|
Relapsed |
92 |
|
Median morphologic blast count in bone marrow (range) |
74 (5–99) |
|
CNS status classification |
|
|
CNS-1 |
85 |
|
CNS-2 |
13 |
|
CNS-3b† |
1 |
|
Unknown |
1 |
Efficacy
- The overall remission rate was 82% at Month 3 (Figure 1) and 98% of these patients had minimal residual disease negative status.
- Among the 65 patients who attained the primary endpoint (CR/CRi within 3 months), the RFS rate was 44% after 5 years, with a median RFS of 43 months.
- Median time to B-cell recovery was 39 months in responders and the probability of B-cell aplasia at 6 and 12 months was 83% and 71%, respectively.
- Among patients in remission, 25% of patients subsequently underwent AlloSCT.
- EFS comparison at 5 years of those without censoring for AlloSCT and those with censoring were 36% and 34%, respectively, with a median EFS of 15 months.
- Median OS was not reached and 5-year OS was 55%.
- There were no significant differences in EFS and OS endpoints between pediatric and young adult patients.
Figure 1. Response rates at Month 3 in patients with R/R B-ALL infused with tisagenlecleucel*
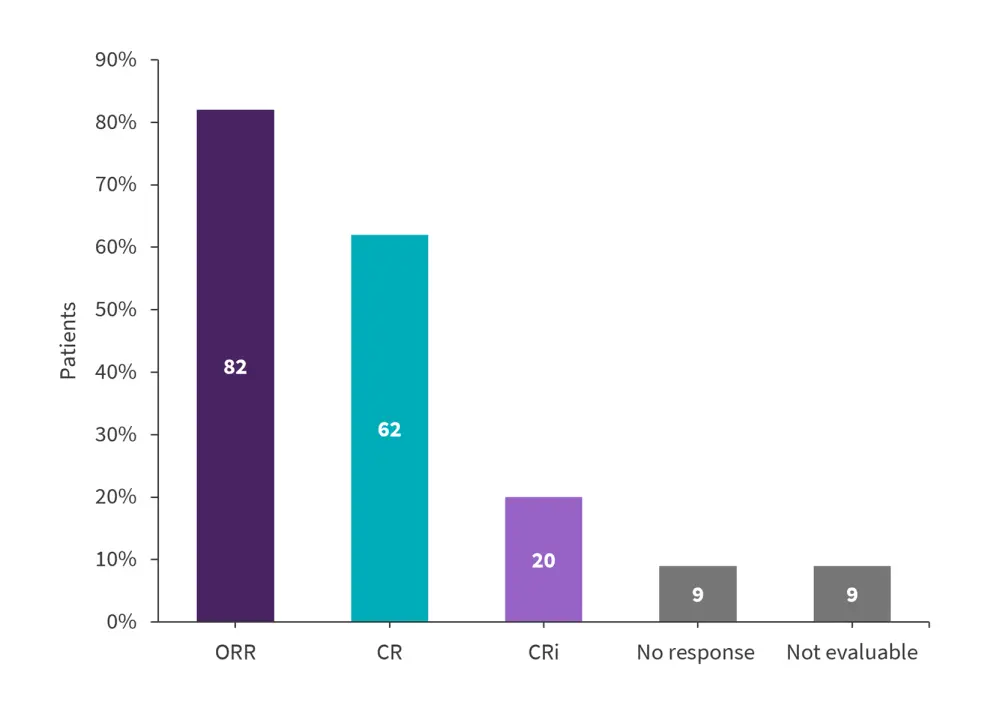
ALL, acute lymphoblastic leukemia; CR, complete remission; Cri, CR with incomplete hematologic recovery; ORR, overall remission rate; R/R, relapsed/refractory.
*Adapted from Rives.2
Safety
Overall, 39% of patients experienced at least one adverse event (AE) of special interest of any grade >1 year post infusion. The most common AEs of any grade and Grade ≥3 were infection in 23 (33%) and 14 (20%) patients, respectively, and hematologic disorders including cytopenias in seven (10%) and four (6%) patients, respectively. Two deaths were reported in remission and no new treatment-related AEs were observed in the 5-year follow up.
Conclusion
This study demonstrated the long-term durable efficacy and safety of tisagenlecleucel in heavily pretreated pediatric and young adult patients with R/R B-ALL. With no new safety events reported and continued efficacy seen at 5 years, tisagenlecleucel therapy continues to show promise as a treatment option for pediatric and adult patients with R/R B-ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

