All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Asparaginase re-exposure after cerebral sinovenous thrombosis in patients aged 1–45 years with ALL
Asparaginase (ASP) is a key part of the treatment of acute lymphoblastic leukemia (ALL) in both adult and pediatric patients. However, many patients experience toxicity associated with ASP treatment such as hypersensitivity reactions. The ALL Hub previously published an article on asparaginase toxicity, discussing the associated issues and potential solutions.
Below, we summarize the results of the NOPHO ALL2008 study (NCT00819351) evaluating ASP re-exposure after cerebral sinovenous thrombosis (CSVT) in pediatric and adult patients with ALL, recently published by Skipper, et al., in EJHaem.1
CSVT is a well-known complication, causing mortality in 1–3% of patients with ALL during ASP therapy. Multiple factors can adversely affect the hemostatic balance in patients with ALL such as ASP, corticosteroids, infection, and high body mass index. However, early termination of ASP therapy is associated with an increased risk of relapse. Data supporting the re-exposure of patients to ASP after CSVT is still lacking; therefore, this study was conducted to examine the frequency of ASP re-exposure after CSVT in patients with ALL.
Study design
This was an observational study including children and adults aged 1–45 years at the time of ALL diagnosis who were treated according to the Nordic Society of Paediatric Haematology and Oncology (NOPHO) ALL2008 protocol between July 2008 and October 2018.
Data were entered into standardized registration forms at three timepoints: (a) at CSVT diagnosis, (b) before ASP re-exposure (in patients re-exposed to ASP), and (c) at last follow-up.
Endpoints
- Primary endpoint was the frequency of ASP re-exposure after CSVT
- Secondary endpoints included:
- clinical characteristics associated with ASP re-exposure;
- CSVT-related outcome; and
- ALL treatment modification following CSVT.
Results
Patient baseline and clinical characteristics
- Overall, 46 patients with CSVT and ALL were included; 31 patients were re-exposed to ASP and 11 were not (Figure 1).
- In total, 3 patients with CSVT died and did not receive any treatment after CSVT diagnosis. Death was due to CSVT, typhlitis and intestinal perforation, cerebral bleeding, and herniation (n = 1 in each category).
- The cumulative incidence of first-time CSVT during ALL therapy was 1.9% and was highest among adolescents.
Figure 1. Study design*
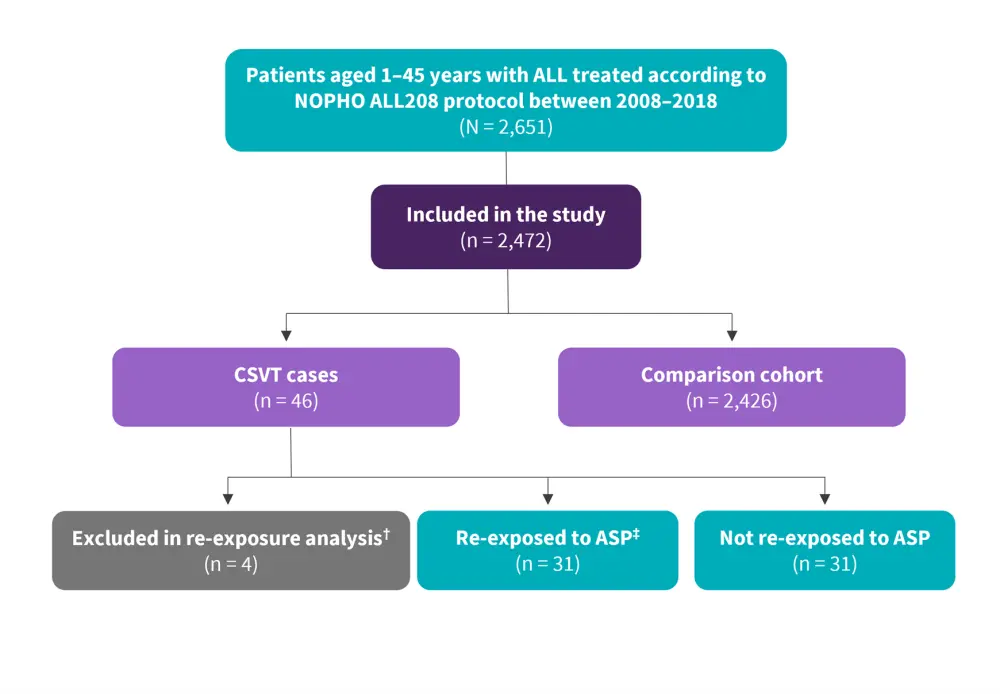
ALL, acute lymphoblastic leukemia; ASP, asparaginase; CSVT, cerebral sinovenous thrombosis.
*Adapted from Skipper, et al.1
†Three patients died and did not receive ALL treatment after CSVT; one had received all ASP doses prior to CSVT.
‡With low-molecular-weight heparin coverage.
- The most frequent locations of thrombosis were the superior sagittal sinus (n = 33), transverse sinus (n = 20), straight sinus (n = 5), and the confluence of sinuses (n = 4).
- The extent of thrombosis in cerebral veins and sinuses at CSVT diagnosis was comparable in later ASP re-exposed and non-re-exposed patients (p = 0.13; Table 1).
Table 1. Clinical characteristics, treatment, and outcome*
|
Characteristics, % (unless otherwise stated) |
Total |
Re-exposure analyses (n = 42)† |
p value |
|
|---|---|---|---|---|
|
|
|
Re-exposed to ASP (n = 31) |
Not re-exposed to ASP (n = 11) |
|
|
Neurological status at diagnosis |
|
|
|
|
|
Seizures |
40 |
48 |
18 |
0.08 |
|
Affected consciousness |
29 |
27 |
27 |
0.97 |
|
Visual disturbance |
12 |
5 |
27 |
0.07 |
|
Headache |
63 |
59 |
72 |
0.41 |
|
Nausea/vomiting |
28 |
34 |
18 |
0.32 |
|
Motor difficulties |
37 |
38 |
36 |
0.93 |
|
Median modified Rankin Scale (IQR) |
2 (1–4) |
2 (1–2.75) |
3 (1.8–3.25) |
0.14 |
|
Imaging at diagnosis |
|
|
|
|
|
Parenchymal lesions |
44 |
48 |
30 |
0.34 |
|
Bilateral lesions |
21 |
19 |
10 |
0.52 |
|
Oedema |
23 |
26 |
0 |
0.09 |
|
Hemorrhage |
31 |
26 |
30 |
0.82 |
|
≥1 venous infarction |
23 |
27 |
10 |
0.27 |
|
CSVT score |
|
|
|
|
|
1 point |
28 |
30 |
22 |
0.13 |
|
2 points |
18 |
26 |
0 |
|
|
3–4 points |
38 |
37 |
44 |
|
|
>4 points |
15 |
7 |
33 |
|
|
ALL treatment |
|
|
|
|
|
Median days on any antithrombotic treatment (IQR) |
232 (183–403) |
240 (184–408) |
280 (175–662) |
0.72 |
|
Truncation of ASP after CSVT |
|
|
|
|
|
≥1 ASP dose omitted |
84 |
81 |
100 |
0.12 |
|
Median number of ASP |
2.5 (1–6.75) |
2 (1–5) |
6 (4–8) |
<0.01 |
|
≥1 intrathecal |
33 |
30 |
36 |
0.7 |
|
Outcome at follow-up |
|
|
|
|
|
Median follow-up (IQR), |
4.5 (2.8–7.4) |
4.6 (2.8–6.8) |
7.59 (3.3–9.5) |
0.43 |
|
Major bleeding during |
4 (0.10) |
1 (0.039) |
1 (0.079) |
0.66 |
|
Imaging at follow-up |
|
|
|
|
|
Recanalisation |
|
|
|
|
|
No recanalisation |
3 |
4 |
0 |
0.48 |
|
Grade I: partial |
29 |
26 |
30 |
|
|
Grade II: complete of ≥1 |
11 |
4 |
20 |
|
|
Grade III: complete of all |
57 |
65 |
50 |
|
|
CSVT score at follow-up |
|
|
|
|
|
No thrombosis, 0 points |
61 |
67 |
56 |
0.43 |
|
1 point |
29 |
29 |
22 |
|
|
2 points |
6 |
5 |
11 |
|
|
3–4 points |
3 |
0 |
11 |
|
|
ALL, acute lymphoblastic leukemia; ASP, asparaginase; BMI, body mass index; CSVT, cerebral sinovenous thrombosis; IR, incidence rate; IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation. |
||||
Re-exposure to ASP
- Patients re-exposed to ASP were significantly earlier in their course of ALL treatment compared with those that were not re-exposed (median 50 days vs 81 days; p = 0.03; Table 1).
- Patients re-exposed to ASP lacked more ASP doses than non-re-exposed patients at CSVT diagnosis (mean 11.2 doses vs 8.4 doses; p = 0.04).
- Frequency of neurologic symptoms at CSVT diagnosis did not differ between patients re-exposed and non-re-exposed to ASP.
- Grade of parenchymal lesions and CSVT score did not impact re-exposure to ASP.
- The neurological status improved in the time between CSVT diagnosis and ASP re-exposure; modified Rankin Scale became normal in 50% of patients, reducing from 2.0 to 0.5 (p < 0.01).
- Partial recanalization was observed in ≥1 sinus in 16 patients, complete recanalization in all occluded sinuses in three patients, no recanalization in three patients, and progression of thrombosis in one patient.
- CSVT score was reduced from Grade III at CSVT diagnosis to Grade II before ASP re-exposure (p < 0.01).
CSVT related outcome
- The median duration of follow-up for patients with CSVT was 4.5 years after diagnosis (Table 1).
- Overall, four patients experienced major bleeding during antithrombotic treatment (bleeding rate, 8.7%).
- Two leukemia relapses occurred in both the re-exposed and non-re-exposed groups.
- Of the 31 patients re-exposed to ASP, two experienced a rethrombosis. One patient had a second CSVT 82 days after the first (15 days after ASP re-exposure) and fully recovered, with a modified Rankin Scale of 0 at the last follow-up. The second patient with multiple thromboses at CSVT diagnosis (pulmonary embolism and bilateral deep vein thrombosis) developed a re-deep vein thrombosis 122 days after CSVT, and 120 days after ASP re-exposure.
Impact on ALL treatment
- The median number of omitted ASP doses per patient with CSVT was 2.5, with significantly more omissions in non-re-exposed patients than in re-exposed patients (p < 0.01; Table 1).
- Eight of 31 re-exposed patients experienced other ASP-related toxicities after ASP re-exposure, including hypersensitivity (n = 4), pancreatitis (n = 2), osteonecrosis (n = 1), and hyperglycemia (n = 1).
- After CSVT, ≥1 scheduled intrathecal chemotherapy was omitted in one-third of patients and the dose of steroids was reduced in one patient.
Neurological outcome and recanalization
- No disability was reported at the last follow-up in 61% of CSVT patients, with no significant difference between re-exposed and non-re-exposed patients (Table 1).
- At a median of 0.5 years after CSVT diagnosis, complete recanalization and partial recanalization were 57% and 40%, respectively, in 35 patients for which data was available.
- Grade of recanalization and CSVT score at the last follow-up were similar among re-exposed and non-re-exposed patients (p > 0.43).
Conclusion
In this study, re-exposure to ASP after CSVT during anticoagulation was found to be safe in patients aged 1–45 years with ALL. Neurological outcomes and recanalization of CSVT were comparable between re-exposed and non-re-exposed patients at the time of the last follow-up, and no factors significantly affected re-exposure to ASP except in the early ALL treatment phase at CSVT diagnosis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


