All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Blinatumomab in newly diagnosed adult patients with ALL in MRD negative remission: Results from ECOG-ACRIN E1910
Introduction
Adults with newly diagnosed acute lymphoblastic leukemia (ALL) achieve a high complete remission rate and measurable residual disease (MRD)-negative status with conventional chemotherapy; however, they often relapse post-induction and have suboptimal survival rates.1
Blinatumomab (blina), a bispecific T-cell engaging antibody that targets CD19, is Food and Drug Administration (FDA) approved for the treatment of children and adults with relapsed/refractory B-ALL and in morphologic complete response (CR) who are MRD positive (≥0.1).1
The ALL Hub has previously published the positive outcome of blina in patients with B-ALL and positive MRD at a minimum threshold of ≥10−4. Below, we report the preliminary results from the phase III ECOG-ACRIN E1910 study (NCT02003222) of blina consolidation in patients with newly diagnosed Philadelphia-negative B-ALL in MRD− remission presented by Litzow, on behalf of the National Cooperative Clinical Trials Network, at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition as a late-breaking abstract.1
Study design
This is an ongoing phase III study of consolidation chemotherapy with/without blina in patients with newly diagnosed Philadelphia-negative B-ALL. In total, 286 patients were randomized to blina + chemotherapy or chemotherapy alone. The study design is outlined in Figure 1.
Figure 1. Study design*
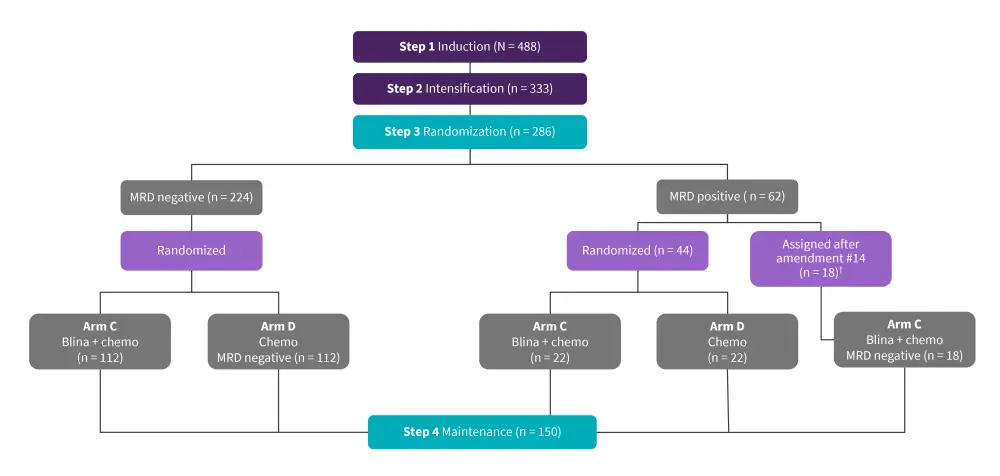
blina, blinatumomab; chemo, chemotherapy; FDA, Food and Drug Administration; MRD, measurable residual disease.
*Adapted from Litzow, et al.
†Following FDA approval of blina for MRD+ disease in March 2018, MRD+ patients were no longer randomized but assigned to arm C.
The objective of the study was to determine if patients who become MRD⁻ (<0.01%) after induction chemotherapy can have improved outcomes with the addition of blina to chemotherapy consolidation compared with patients who received chemotherapy alone.
Results
- Overall, 488 patients with a median age of 51 years (range, 30–70 years) were enrolled in Step 1 induction therapy.
- In total, 224 MRD⁻ patients were randomized, with 112 patients in each arm and 22 patients in each arm proceeding to allogeneic bone marrow transplant.
- The CR/CR with incomplete hematologic recovery (CRi) rate after induction chemotherapy was 81%; the CR rate was 75% and CRi rate was 6%.
- In a third interim efficacy analysis of MRD⁻ patients, 17 patients in the blina arm and 39 in the control chemotherapy arm had died.
- The upper limit for efficacy analysis was crossed in favor of blina with a significant improvement in overall survival for the blina arm (median OS, not reached vs 71.4 months; Hazard ratio, 0.42; 95% confidence interval [CI], 0.24–0.75; two-sided p = 0.003).
- The median follow-up was 43 months and no significant safety signals were reported.
Conclusion
The addition of blina to consolidation chemotherapy has shown better survival outcomes vs chemotherapy alone in patients with newly diagnosed B-ALL who were MRD⁻ after intensification, with no new safety concerns reported. Based on these findings, the authors suggest that the addition of blina to consolidation chemotherapy represents a new standard of care for adult patients with MRD⁻ Philadelphia-negative B-ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


