All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Blinatumomab induces cures in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia
Due to the high risk of relapse, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered to be the best treatment option for adult patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) who have achieved clinical remission after chemotherapy but remain minimal residual disease (MRD) positive. Yet, transplantation outcomes remain suboptimal in these patients, and transplantation itself may not be suitable for those who are older or have co-morbidities.
In the multi-national, single-arm BLAST study (NCT01207388), recently published in Leukemia & lymphoma, ALL Hub steering committee member Nicola Gökbuget and colleagues1 investigated long-term outcomes of blinatumomab administration in adults with BCP-ALL in remission with MRD after chemotherapy, with a minimum patient follow-up of 5 years.
Study design
The inclusion criteria for this study were adults aged ≥ 18 years with BCP-ALL in hematologic complete remission (CR; < 5% blasts in the bone marrow) and measurable MRD (≥ 10-3 ≥ 2 weeks after last chemotherapy) after ≥ 3 blocks of intensive chemotherapy. Blinatumomab was administered as follows: 15 µg/m2/day for up to four 6-week cycles (4 weeks continuous infusion, 2 weeks off). Allo-HSCT was allowed any time after Cycle 1.
MRD assessments were performed by a central reference laboratory at baseline, at the end of each treatment cycle, and during efficacy follow-up using real-time quantitative polymerase chain reaction (qRT-PCR). The threshold for complete MRD response was defined as 10-4 after blinatumomab induction.
Patient characteristics and disposition
The median age of patients was 45 years (range, 18–76), with almost half of the patients (47%) having MRD ≥ 10-2 and about a third of patients (35%) in second or later remission.
Results
In the primary analysis, there were a total of 116 patients who received blinatumomab, of which 110 were analyzed for overall survival. Median survival was 36.5 months (95% CI, 22.0–not reached [NR]), with a median follow-up of 59.8 months. Median OS was best in patients receiving blinatumomab in first remission, 41.2 months (95% CI, 23.5–NR), while it was only 23.1 months (15.4–NR) for patients in second or later remission (log-rank p = 0.40).
In the final analysis, 74 patients received HSCT in continuous complete remission (CCR) after blinatumomab, with complete MRD response as shown in Figure 1. The characteristics of adults with BCP-ALL and MRD, who received HSCT in CCR after blinatumomab treatment, are shown in Table 1.
Figure 1. Patient disposition, use of HSCT, and complete MRD responses after blinatumomab1
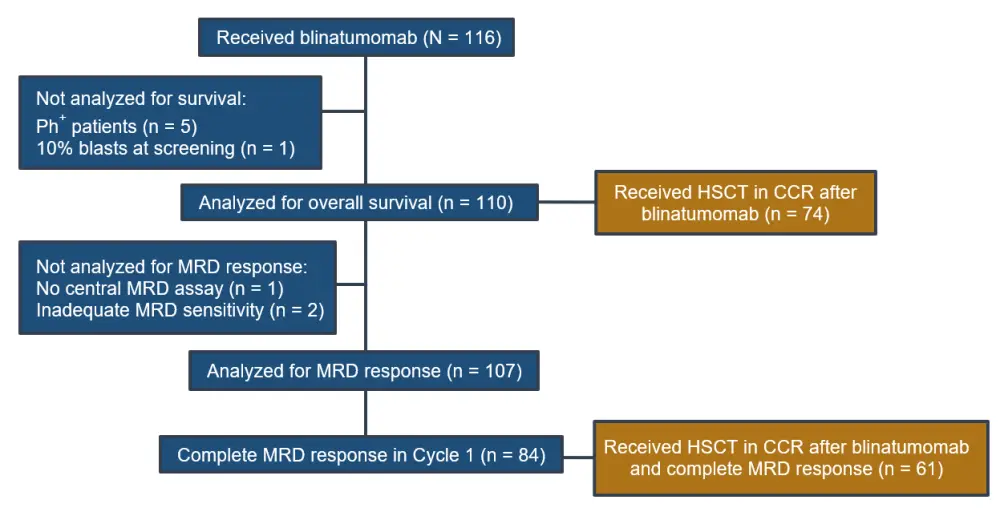
CCR, continuous complete remission; HSCT, hematopoietic stem cell transplantation; MRD, minimal residual disease; Ph+, Philadelphia chromosome–positive.
Of the 74 patients who received HSCT in CCR after blinatumomab, 61 patients (82%) achieved a complete MRD response. Thirty-six patients have not received HSCT in CCR, of which 23 patients achieved a complete molecular remission after blinatumomab (see Table 2).
Table 1. Characteristics of adults with BCP-ALL and MRD who received HSCT in CCR after blinatumomab treatment1
|
BCP-ALL, B-cell precursor acute lymphoblastic leukemia; CCR, continuous complete remission; HSCT, hematopoietic stem cell transplantation; MRD, minimal residual disease. *Complete MRD response was defined as no target amplification with a minimum sensitivity of 10-4 at the end of Cycle 1. †Included one haploidentical donor. |
|
|
Characteristic |
Received HSCT (n = 74) |
|---|---|
|
Baseline characteristics |
|
|
Median age, years (range) |
42 (18–67) |
|
Prior relapses, n (%) |
|
|
0 (first remission) 1 (second remission) |
55 (74) 19 (26) |
|
After blinatumomab treatment |
|
|
Complete MRD response, n (%)* |
|
|
Yes No Not determined |
61 (82) 11 (15) 2 (3) |
|
Donor relationship, n (%) |
|
|
Related Cord Unrelated Unknown |
19 (26)† 4 (5) 49 (66) 2 (3) |
|
Matching of unrelated HSCT, n (%) |
|
|
Matched, sibling Matched, unrelated Mismatched Missing |
17 (23) 20 (27) 25 (34) 12 (16) |
|
HSCT conditioning regimen, n (%) |
|
|
Myeloablative Reduced/non-myeloablative Missing |
55 (74) 14 (19) 5 (7) |
As shown in Figure 2, at the end of the follow-up study, 30 of 74 patients (40.5%) and 7 of 36 patients (19.4%) with and without HSCT in CCR, respectively, were alive without relapse. Moreover, 27 of 74 patients (36.5%) and 3 of 36 patients (8.3%) patients with and without HSCT in CCR, respectively, died without relapse. Among complete MRD responders, 28 of 61 patients (45.9%) and 7 of 23 patients (30.4%) with and without HSCT in CCR, respectively, were alive without relapse. Among those complete MRD responders, 20 of 61 patients (32.8%) with HSCT and 2 of 23 patients (8.7%) without HSCT died without relapse.
Figure 2. Outcomes with or without HSCT in CCR after blinatumomab at the final visit at 5 years1
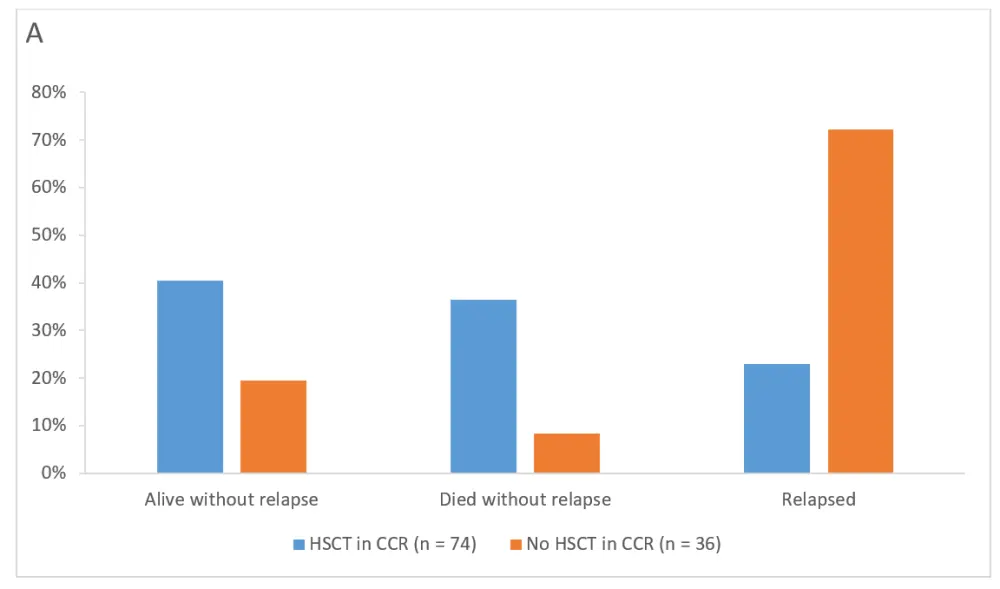
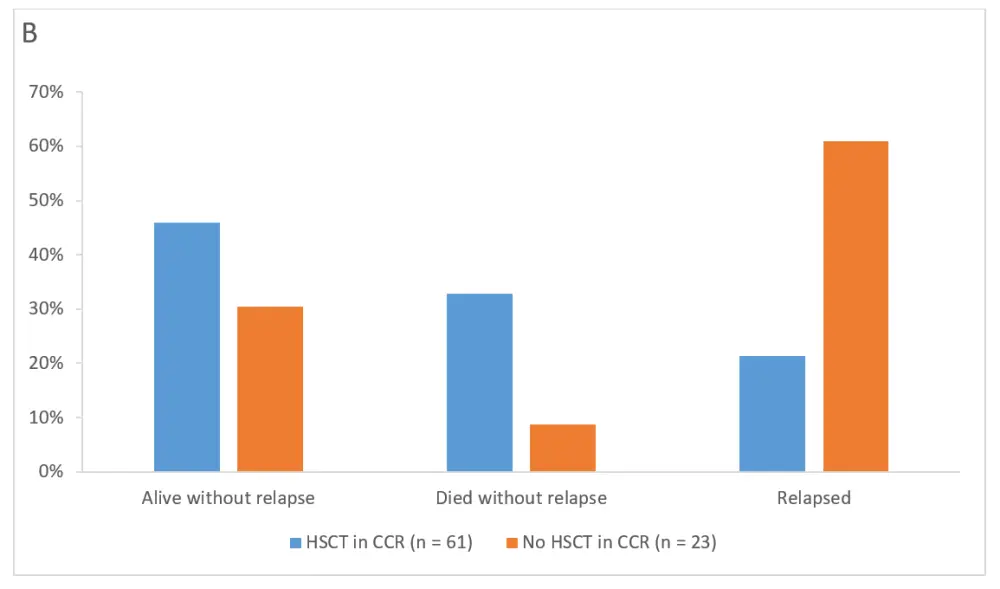
A All patients at final visit (5 years). B Complete MRD responders at final visit (5 years)
CCR, continuous complete remission; HSCT, hematopoietic stem cell transplantation.
Table 2. Change in MRD level from baseline to the end of Cycle 1 in patients not achieving a complete MRD response (n = 23)1
|
MRD, minimal residual disease; NA, not applicable. *The non-missing minimum MRD result was used in case multiple MRD measurements were taken during Cycle 1. †The latest one prior to blinatumomab infusion was used in case of multiple MRD measurements were taken at baseline visit. |
|||||||
|
Cycle 1 MRD* value |
|||||||
|---|---|---|---|---|---|---|---|
|
Baseline MRD† |
−5 |
−4 |
−3 |
−2 |
−1 |
NA |
Total |
|
NA |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
|
−5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
−4 |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
|
−3 |
1 |
6 |
2 |
0 |
1 |
0 |
10 |
|
−2 |
0 |
5 |
2 |
0 |
1 |
0 |
8 |
|
−1 |
1 |
0 |
0 |
0 |
0 |
2 |
3 |
|
Total |
2 |
13 |
4 |
0 |
2 |
2 |
23 |
Conclusion
In conclusion, the data from this multi-national, single-arm clinical trial found that blinatumomab showed promising long-term outcomes in adults with BCP-ALL and MRD after a minimum follow-up of 5 years, suggesting that blinatumomab could be considered a potential curative treatment in patients who achieve complete MRD response after the first cycle of blinatumomab. Furthermore, these results indicate that cure could be possible in some patients without allo-HSCT. The long-term follow-up of adults with BCP-ALL is the strength of the study, whereas the single-arm design and lack of assessment of the safety and efficacy of HSCT following treatment with blinatumomab are limitations.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

