All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Blinatumomab prior to CD19 CAR T-cell therapy for the treatment of relapse/refractory ALL
Regulatory agencies in Europe and the United States have approved two agents that target CD19 for the treatment of relapsed/refractory acute lymphoblastic leukemia in children and young adults: blinatumomab and anti-CD19 chimeric antigen receptor (CAR) T-cell tisagenlecleucel. It is known that both agents are associated with CD19 loss or downregulation. However, certain patients may receive both during the course of their treatments. Therefore, there is a high need to understand whether these treatments can be sequenced without inducing the development of resistance to CD19 targeting.
During 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Nirali Shah presented a talk on the impact on event-free survival (EFS) of using blinatumomab before CAR T-cell therapy.
Study design and patient baseline characteristics
- This retrospective study included 420 patients, and the primary objectives were EFS and relapse-free survival (RFS) at 6 months following CD19 CAR infusion.
- The secondary objectives were EFS and RFS at 12 months, as well as to evaluate any changes in CD19 expression.
- Patients who were included were ≤ 25 years at time of diagnosis. The median age at infusion was 12.4 years (interquartile range [IQR], 7–17.1 years), with a median follow-up following infusion of 2.3 years (IQR, 1.6–3.3 years).
- Out of the patients included in this study, 17.9% had previous exposure to blinatumomab and 57.3% achieved a complete response (CR). The median time to last blinatumomab infusion was 129 days (IQR, 79–304 days).
- Of the three different CAR T-cell constructs used, ~20% of patients received commercial tisagenlecleucel.
- Statistical assessment was carried out for all groups shown in Table 1, but a significant difference was only found between the group treated with blinatumomab and the blinatumomab-naïve patients for KMT2Ar prevalence (p = 0.03).
Table 1. Baseline patient characteristics1
|
Blina, blinatumomab; CAR, chimeric antigen receptor; CNS3, central nervous system status 3; EM, extramedullary; MRD, measurable residual disease. |
|||
|
Pre-CAR disease status |
All (N = 420) |
Prior blina (n = 75) |
No blina (n = 345) |
|---|---|---|---|
|
M1 or MRD-negative marrow, % |
51.6 |
53.3 |
51.3 |
|
M2/M3 marrow, % |
48.3 |
46.6 |
48.7 |
|
CNS3, % |
1.1 |
0.0 |
1.2 |
|
Active EM disease, % |
5.2 |
8.0 |
4.6 |
|
Circulating blasts, % |
15.9 |
18.7 |
15.3 |
|
KMT2Ar, % |
17.0 |
14.7 |
6.7 |
Key points
- A total of 412 patients were available for evaluation of the response to CAR T-cells. Of these patients, 91% achieved CR (Table 2) and 88.1% were measurable residual disease-negative.
- At the time of analysis, 234 patients remained alive and in CR, and the relapse rate was 39.8%.
- Following CD19 CAR T-cell therapy, patients who had received blinatumomab previously were more likely to have residual disease.
- Of the patients that had received blinatumomab previously, 18.3% were non-responders, compared with only 7% in the group that had not received blinatumomab previously.
Table 2. Response to anti-CD19 CAR T-cell therapy1
|
Blina, blinatumomab; CAR, chimeric antigen receptor; CR, complete response. |
|||
|
Previous therapy |
CD19 CAR T-cell therapy response |
||
|---|---|---|---|
|
CR |
Non-CR |
Total |
|
|
Blina, % |
14 |
3 |
17 |
|
No blina, % |
77 |
6 |
83 |
|
Total (N = 412), % |
91 |
9 |
100 |
- Outcome was significantly worse in the blinatumomab group for both RFS (p = 0.027) and EFS (p = 0.0034) compared with patients without previous exposure.
- The median RFS for the group exposed to blinatumomab was 20.3 months vs 44.9 months in the patients that had not received blinatumomab treatment previously, as shown in Table 3.
Table 3. RFS and EFS at 6 and 12 months1
|
Blina, blinatumomab; EFS, event-free survival; NE, not estimable; RFS, relapse-free survival. |
||||||
|
Previous therapy |
6-month RFS, % |
12-month RFS, % |
Median RFS, months |
6-month EFS, % |
12-month EFS, % |
Median EFS, months |
|---|---|---|---|---|---|---|
|
Blina |
63.4 |
57.5 |
20.3 |
49.7 |
46.7 |
5.8 |
|
No blina |
81.1 |
69.2 |
44.9 |
72.1 |
59.6 |
22.6 |
- Prior to CD19 CAR T-cell infusion in the blinatumomab group, 13% of patients had either negative, dim, or partial CD19 expression. In the group that had not received prior blinatumomab, the incidence was only 6.2%.
- When CD19 expression was examined before and after blinatumomab treatment, 11.5% of patients were found to have switched to dim CD19 expression.
Conclusion
This study found that treatment with blinatumomab before CD19 CAR T-cell infusion was associated with significantly poorer outcomes in terms of RFS and EFS. Leukemic blast cells of patients who had been treated with blinatumomab showed more frequently a dim or no expression of CD19 antigens prior to CAR T-cell infusion and patients were at increased risk of not responding to CD19 CAR T-cell therapy.
The authors suggested that future studies should investigate whether there is an inherent risk for resistance to CD19 targeting in patients relapsing after blinatumomab and whether patients with prior blinatumomab might benefit from consolidation with hematopoietic stem cell transplantation.
Update
Following up from the talk at 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Regina Myers and colleagues published an article in the Journal of Clinical Oncology with an update on this study.1
Results
Survival outcomes
For the whole cohort, the median follow-up was 30.1 months. Patients that were treated with blinatumomab (blina) but did not achieve a complete response (CR) showed a shorter relapse free survival (RFS), event free survival (EFS) and overall survival (OS) compared with blina-CR or blina-naïve (Table 1).
Table 1. CD19 response and survival outcomes at 24 months*
|
Blina, blinatumomab; CAR, chimeric antigen receptor; CI, confidence interval; CR, complete response; EFS, event free survival; OS, overall survival; RFS, relapse free survival. |
||||
|
Outcome |
All |
Blina-No CR |
Blina-CR |
Blina-naïve |
|---|---|---|---|---|
|
Median follow-up |
30.1 (21.0–48.1) |
32.4 (23.7−42.9) |
24.2 (18.3−36.0) |
30.8 (22.1−48.9) |
|
24-month RFS, % |
56.4 (50.8−61.6) |
22.7 (7.3−43.2) |
56.6 (37.9−71.6) |
58.6 (52.5−64.1) |
|
24-month EFS, % |
47.4 (42.3−52.3) |
13.7 (4.5−28.0) |
51.3 (34.2−66.1) |
50.2 (44.6−55.6) |
|
24-month OS, % |
65.1 (60.0−69.6) |
37.9 (21.0−54.8) |
76.5 (59.4−87.1) |
66.3 (60.8−71.3) |
Disease burden
A high disease burden was found to be associated with poor survival in this study. Blina use added to the negative impact of high disease burden for EFS and RFS but not OS. For blina non-responders, high disease burden prior to chimeric antigen receptor (CAR) T-cell infusion increased the chance of a poor outcome.
HSCT outcomes
Following CD19 CAR T-cell therapy, 146 patients went on to receive a hematopoietic stem cell transplant (HSCT). Of these, 92 received HSCT within a year after CD19 CAR therapy, 13 had a previous transplant, and six had been given blina before. The median time from CAR T-cell infusion to HSCT was 93 days (range, 42–301 days).
Survival outcomes following HSCT were:
- 2-year OS = 78.3% (95% confidence interval [CI], 67.6–85.8)
- 2-year EFS = 70.8% (95% CI, 59.5–79.5)
- 2-year RFS = 82.6% (95% CI, 71.1–89.8)
A relapse following HSCT occurred in 13.4% of patients, in two patients this was a relapse after a second HSCT.
CD19 modulation
Pre-CAR CD19 expression analysis was available for 414 patients. In blinatumomab exposed patients, dim CD19 or partial expression was seen more frequently compared with blina-naïve (13.3% vs 6.5%; p = 0.06). For blina patients with a strong CD19 positive expression before CAR treatment (n = 376), CR rates were 82.5%, a decrease compared with 92.9% in the blina-naïve patients (p = 0.01). Figure 1 shows a breakdown of relapse phenotype in both blina-naïve and blina-exposed patients, demonstrating that the phenotype at relapse was similar between the two groups.
Figure 1. CD19-positive expression in patients pre-CAR (n = 382)
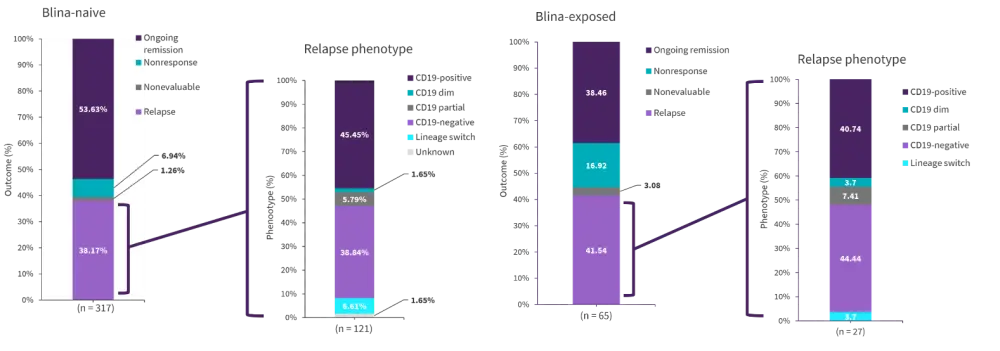
Blina, blinatumomab.
*Adapted from Myers, et al.1
Compared with 95% of the blina-naïve patients, 62.5% of the blina-exposed patients reached CR in patients with CD19-dim or partial expression (p = 0.06). All five of the blina-exposed patients who achieved CR subsequently relapsed. Of the 28 patients with CD19-dim expression before CAR T-cell infusion, 60.7% experienced a relapse or nonresponse. Patients who were given blina and then became CD19-dim showed particularly poor outcomes (Figure 2).
Figure 2. CD19-dim expression in patients pre-CAR (n = 29)*
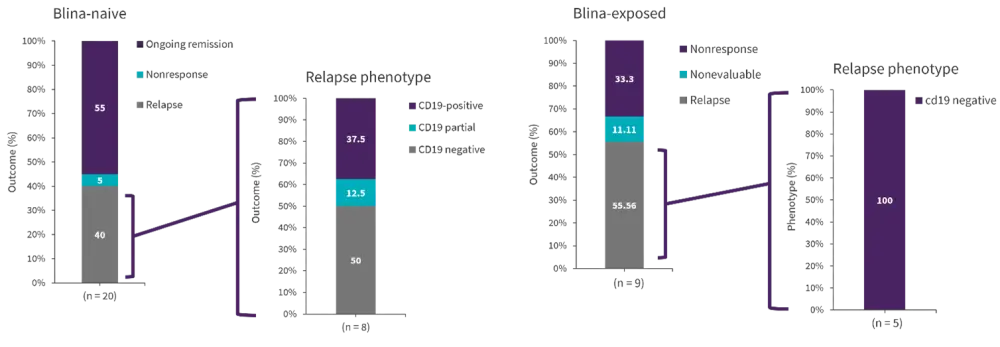
Blina, blinatumomab.
*Adapted from Myers, et al.1
Conclusion
While blina exposure does not preclude a response to CD19 CAR T-cell therapy, blina-exposed non-responders were found to have poorer outcomes than blina-naïve or previous blina-responders. Blina exposed non-responders demonstrated lower CR rates coupled with increased incidence of relapse compared with blina-naïve patients. Blina use may result in CD19 downregulation, in patients where CD19 expression was reduced following treatment CD19 negative disease relapse was common.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

