All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
CNS involvement at diagnosis and its association with CNS toxicities in childhood ALL
Central nervous system (CNS) leukemia is clinically characterized by the presence of leukemic cells in cerebrospinal fluid (CSF). Routine diagnosis by cytomorphology (CM) has been linked to an increased risk of CNS toxicities and early posterior reversible encephalopathy syndrome (PRES) in recent studies. Flow cytometric immunophenotyping (FCI), which is a less commonly used diagnostic tool, can detect low levels of leukemic blasts in the CSF and has a higher sensitivity than CM.
CNS-directed chemotherapy is administered to all patients with acute lymphoblastic leukemia (ALL) at diagnosis as a preventative measure against CNS relapse, regardless of CNS involvement. On the other hand, enhanced intrathecal chemotherapy, which has been associated with CNS toxicities, is given to patients with ALL on diagnosis of CNS leukemia. There is still uncertainty as to whether CNS toxicities are due to directed CNS prophylactic measures, or a result of the presence of leukemic cells in the CNS.
Here, we summarize a recently published article by Anastasopoulou et al.1 in Blood, investigating the role of minimal CNS involvement at diagnosis on the risk of CNS toxicities by FCI in pediatric patients with ALL.
Methods
Patients included in the analysis were
- children aged 1–17.9 years;
- diagnosed with ALL between 2008 and 2015;
- treated according to the Nordic Society of Paediatric Haematology and Oncology (NOPHO) ALL2008 protocol; and
- had diagnostic data on CNS involvement by both CSF by FCI and CM.
CNS involvement by CM categorized patients into three groups:
- CNS1: no CNS involvement
- CNS2: <5 /μl leukemic cells and blasts in the CSF
- CNS3: ≥5 /μl leukemic cells and blasts in the CSF or clinical signs of CNS involvement
CNS involvement by FCI classified patients into two groups:
- positive for CNS involvement (CNSflow+): ≥10 leukemic blasts in the CSF by FCI
- negative for CNS involvement (CNSflow−): <10 leukemic blasts in the CSF by FCI
The three groups of CNS toxicities in patients were:
- all CNS toxicities (including any associated toxicity)
- seizures (both isolated and secondary to CNS toxicities)
- PRES
The study endpoints were the association between CNS leukemia by FCI at diagnosis and the risk of CNS toxicities; the association of CNSflow+ with CNS toxicity was also tested.
Results
Baseline clinical characteristics and toxicities
Overall, 370 patients were included in the study. Within the CNS1 group (n = 320), 256 were CNSflow− and 64 were CNSflow+, as seen in Figure 1.
Figure 1. Patient classification of CNS involvement at diagnosis based on combined CM and CSF FCI data*
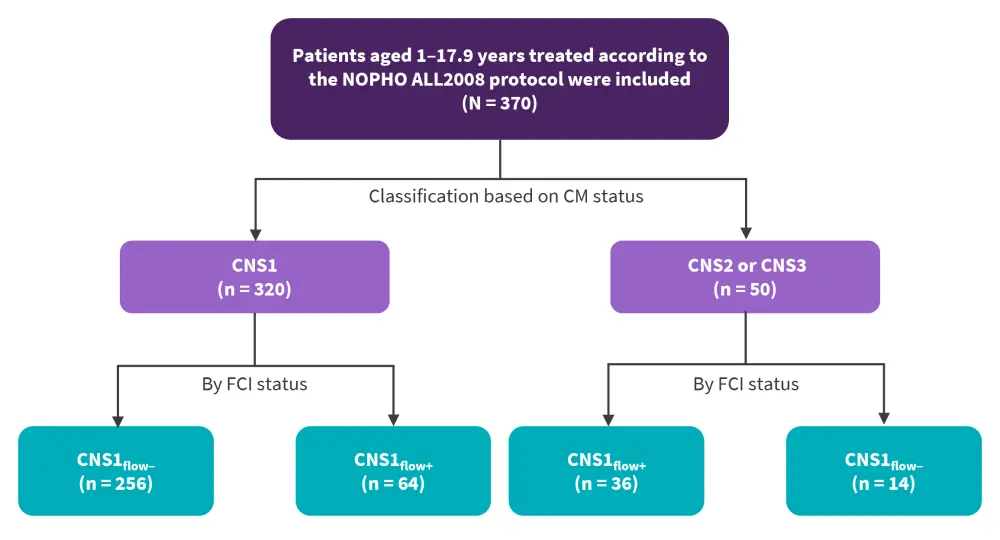
ALL, acute lymphoblastic leukemia; CM, cytomorphology; CNS, central nervous system; FCI, flow cytometric immunophenotyping; NOPHO, Nordic Society of Paediatric Haematology and Oncology.
*Data from Anastasopoulou, et al.1
At baseline, 38 patients overall reported at least one type of CNS toxicity, with 22 of these cases occurring with seizures. Of these 22, 16 cases were associated with PRES. In CNS1 patients who were CNSflow+, there was a total of 33 CNS toxicity cases; 18 presented with seizures and 14 of these related to PRES.
Clinical characteristics that were significantly higher at diagnosis in CNS1 patients with CNSflow+ included white blood cell count and T-cell immunophenotypes (p < 0.001; Table 1).
Table 1. Clinical characteristics at diagnosis of CNS1 patients*
|
BCP, B-cell precursor; CNS, central nervous system; FCI, flow cytometric immunophenotyping; WBC, white blood cells. |
||||
|
Characteristic, % (unless |
CNS1 |
CNSflow− |
CNSflow+ |
p value |
|---|---|---|---|---|
|
Age median and range |
4.0 (1.0–17.0) |
4.0 (1.0–17.0) |
4.0 (1.0–16.0) |
— |
|
Sex |
|
|
|
— |
|
Male |
54.1 |
56.6 |
43.8 |
|
|
Female |
45.9 |
43.4 |
56.3 |
0.064 |
|
WBC, × 109/L |
|
|
|
|
|
<100 |
90.9 |
95.3 |
73.4 |
|
|
>100 |
9.1 |
4.7 |
26.6 |
<0.001 |
|
Immunophenotype |
|
|
|
|
|
BCP |
89.4 |
93.0 |
75.0 |
|
|
T cell |
10.6 |
7.0 |
25.0 |
<0.001 |
|
Induction therapy† |
|
|
|
|
|
Prednisolone |
84.7 |
89.7 |
64.5 |
|
|
Dexamethasone |
15.3 |
10.3 |
35.5 |
<0.001 |
|
Stratification into block treatment at the end of induction |
|
|
|
|
|
Non-block treatment |
85.0 |
85.9 |
81.3 |
|
|
Block treatment |
15.0 |
14.1 |
18.8 |
0.348 |
The univariate analysis in all CNSflow+ patients, which included both patients who received (CNS1) and did not receive enhanced intrathecal treatment (CNS2 and CNS3), revealed an increased risk for seizures (hazard ratio [HR], 2.72; 95% confidence interval [CI], 1.18–6.28; p = 0.019] and PRES (HR, 2.72; 95% CI, 1.18–6.28; p = 0.019) when compared with CNSflow− patients. After adjusting for the type of induction therapy in the multivariate analysis, patients with CNSflow+ still had a significant risk for PRES (HR, 2.72; 95% CI, 1.18-6.28; p = 0.019).
Among CNS1 patients who did not receive enhanced intrathecal treatment, univariate analysis showed that CNSflow+ status alone was associated with an increased risk of all CNS toxicities, seizures, and PRES when compared with toxicities in CNSflow- patients . In the multivariate analyses, which adjusted for age and subsequently induction therapy, CNS1flow+ status was still a significant risk factor for all CNS toxicities (Table 2).
Table 2. Risk of CNS toxicity in CNS1 patients*
|
CI, confidence interval; CNS, central nervous system; HR, hazard ratio; PRES, posterior reversible encephalopathy syndrome. |
|||||
|
Toxicity, % (unless |
Controls |
Cases |
Univariate HR (95% CI; p value) |
Multivariate HR† (95% CI; p value) |
Multivariate HR‡ (95% CI; p value) |
|---|---|---|---|---|---|
|
All CNS toxicities, n |
287 |
33 |
|
|
|
|
CNSflow- |
81.5 |
66.7 |
Ref. |
Ref. |
Ref. |
|
CNSflow+ |
18.5 |
33.3 |
2.08 (1.01–4.28; 0.048) |
2.35 (1.13–4.87; 0.022) |
1.90 (0.88–4.10; 0.101) |
|
|
|
|
|
|
|
|
Seizures, n |
287 |
18 |
|
|
|
|
CNSflow- |
81.5 |
55.6 |
Ref. |
Ref. |
Ref. |
|
CNSflow+ |
18.5 |
44.4 |
3.34 (1.32–8.47; 0.011) |
3.83 (1.50–9.76; 0.005) |
3.33 (1.26–8.82; 0.016) |
|
|
|
|
|
|
|
|
PRES, n |
287 |
16 |
|
|
|
|
CNSflow- |
81.5 |
43.8 |
Ref. |
Ref. |
Ref. |
|
CNSflow+ |
18.5 |
56.3 |
5.30 (1.98–14.24; < 0.001) |
6.13 (2.26–16.64; < 0.001) |
4.85 (1.71–13.75; 0.003) |
Conclusion
This study demonstrated that minimal CNS involvement without enhanced CNS chemotherapy, as determined by FCI, is significantly associated with an increased risk for CNS toxicities, with the highest risk seen for PRES. Despite the small cohort, both univariate and multivariate analyses reported a positive association between CNS leukemia and risk of CNS toxicities. These results can be a foundation for future studies on the allocation of CNS prophylactic measures.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

