All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Coadministration of CD19- and CD22-targeted CAR T cells in children with R/R B-cell ALL
Introduction
Relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (ALL), historically characterized by a poor prognosis and increased morbidity, remains a challenge to treat in children. Tisagenlecleucel, an autologous CD-19-directed chimeric antigen receptor (CAR) T-cell therapy, showed significant efficacy and a tolerable safety profile in children with R/R B-ALL in a primary analysis of the ELIANA phase II trial (NCT02435849).1 Preclinical research has also demonstrated that CD19-targeting CAR T cells can downregulate CD22 expression in a subset of tumor cell line models.
Recently, Wang et al. published outcomes from a phase II study of coadministration of CD19- and CD22-directed CAR T-cell therapies in childhood B-ALL in Journal of Clinical Oncology.2 Considering the fundamental treatment principle of ALL that combination therapy prevents drug resistance and a preclinical model demonstrating that simultaneous CD19-/CD22 targeting may reduce the risk of antigen loss, the authors investigated whether coadministration of CD19- and CD22-targeted CAR T cells could improve efficacy.
Study design
This was a phase II, open-label, multicenter study in children aged ≤21 years with R/R B-ALL. Patients received CD19-/CD22-CAR T-infusion (n = 225) and were separated into three cohorts (Figure 1).
Figure 1. Study design*
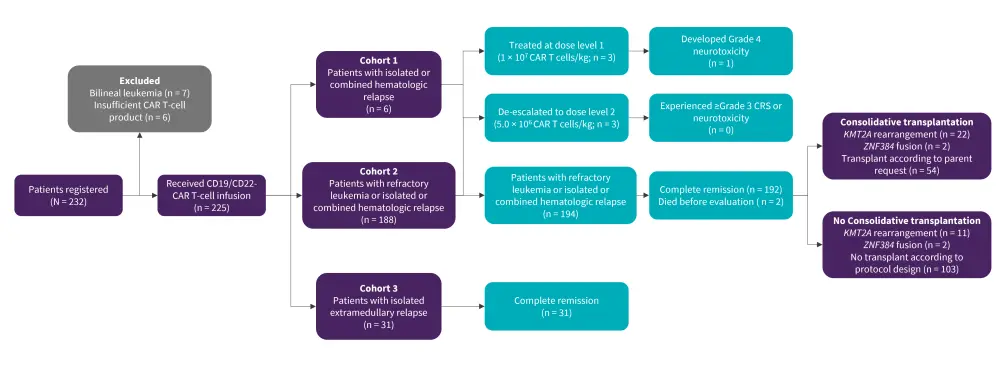
CAR, chimeric antigen receptor.
*Adapted from Wang, et al.2
CD31 T lymphocytes were collected from peripheral blood (1–2 mL/kg) and CAR T cells were manufactured at the Shanghai Children’s Medical Center. CD19-/CD22-specific CAR T cells were cultured separately and pooled together (1:1).
The primary endpoint was recommended phase II dose of combined CD19-/CD22-CAR T cells, CAR T-cell related adverse effects, complete remission rate at Day 28 post-infusion, event-free survival (EFS), and overall survival at Month 12 with or without consolidative transplantation. Exploratory analyses were performed on the effect of sustained B-cell aplasia.
Baseline characteristics
Overall, 225 patients were infused with CD19-/CD22-specific CAR T cells with a median age of 7.6 years. Patient baseline characteristics are summarized in Table 1.
Table 1. Selected baseline and clinical characteristics*
|
CAR, chimeric antigen receptor. |
|
|
Characteristic, % (unless otherwise stated) |
All patients (N = 225) |
|---|---|
|
Median age (range), years |
7.6 (0.8–19.6) |
|
Disease status, n |
|
|
Refractory/hematologic relapse |
194 |
|
Isolated extramedullary relapsed |
31 |
|
The median time from enrollment to infusion (range), days |
7 (6–12) |
|
The median dose of combined CD19- and CD22-CAR T-cell (IQR), per Kg |
5.6 ×106 (4.1–7.6 ×106) |
|
The median dose of CD19-CAR T cells |
2.7 ×106 (1.9–3.7 ×106), |
|
The median dose of CD22-CAR T cells |
2.8 ×106(2.1–4.0 ×106) |
Primary outcomes
- Complete remission was achieved in 99% of the patients with 100% minimal residual disease-negative status.
- With a median follow-up of 11 months, 43 patients relapsed (24 with CD19+/CD22+ relapse, 16 with CD19–/CD22+, one with CD19–/CD22–, and two unknown).
- The 12-month EFS was 73.5% and 69.2% (transplant and non-transplant, respectively), after censoring 78 patients for consolidative transplantation, and the 12-month overall survival was 87.7%.
Secondary outcomes
- B-cell aplasia occurred in 181 patients (peripheral blood/bone marrow) and the median time to normal B-cell recovery (≥1%) was 74.0 days.
- The cumulative incidence of loss of B-cell aplasia by 6 months post infusion was 59.8%.
- EFS for patients who had persistent B-cell aplasia at 2 months after infusion and beyond showed a steady improvement at 2, 3, 4, and ≥6 months (77.0%, 88.7%, 97.4, and 100%, respectively).
- Among the 116 patients who received only coadministration of CD19-/CD22-CAR T cells and did not undergo consolidative transplantation, minimal residual disease before CAR T-cell treatment (15% vs 54.6%; p = 0.04), M1 bone marrow status (76.3% vs 58.3%; p = 0.05), and persistent B-cell aplasia for ≥6 months were significantly associated with favorable 12-month EFS (100% vs 47.2%; p < 0.001).
- The multivariate analysis showed a positive association between EFS, consolidative transplantation (p = 0.07), and persistence of B-cell aplasia for ≥6 months post infusion (p < 0.001).
CAR T-cell persistence
- In the 76 patients tested, expansion occurred earlier for CD19-CAR T cells vs CD22-CAR T cells (7.3 days vs 10.9 days; p = 0.0013).
- CD19-CAR T cells had more robust and longer expansion duration than CD22-CAR T cells.
- Among the 21 patients who relapsed,
- patients with CD19+/CD22+ relapse (n = 11) lost CD19− and CD22-CAR T-cell persistence at relapse;
- patients with CD19−/CD22+ relapse (n = 9), four lost CD19-CAR T-cell persistence, but all nine lost CD22-CAR T cells at relapse; and
- patients with CD19−/CD22− relapse did not lose CD19- but lost CD22-CAR T-cell persistence at relapse.
Isolated extramedullary relapse and safety
- Overall, 31 patients were treated for isolated extramedullary relapse.
- All patients experienced complete remission without local irradiation.
- With a median follow-up of 13.3 months, three of the 10 patients treated for CNS relapse had adverse events (two CNS relapses and one fatal neurotoxicity) and one of the 20 patients treated for testicular relapse developed hematologic relapse, resulting in a 12-month EFS of 68.6% and 95.0%, respectively.
- The patient with combined testicular and CNS relapse remained in complete remission for 14.4 months.
Toxicities that occurred within 4 weeks of infusion are summarized in Table 2.
Table 2. Safety outcomes in patients with R/R B-ALL infused with CD19-/CD20 CAR T-cells*
|
CAR, chimeric antigen receptor; CRS cytokine release syndrome; B-ALL, B-cell acute lymphoblastic leukemia; EM, extramedullary; R/R, relapsed/refractory. ‡One patient with hematologic relapse died of neurotoxicity at 4 days after infusion. One patient with a second isolated CNS relapse died of neurotoxicity at 1.8 months after infusion. |
|||
|
Toxicity, % (unless otherwise stated) |
All patients |
R/R disease |
Isolated EM relapse |
|---|---|---|---|
|
CRS |
|
|
|
|
Any |
88 |
88.1 |
87.1 |
|
Grade 3 and 4 |
28.4 |
27.3 |
35.5 |
|
Grade 5† |
0.4 |
0.5 |
0 |
|
Median time to onset of cytokine release syndrome, days |
1 |
1 |
1 |
|
Median duration of cytokine release syndrome, days |
5 |
5 |
5 |
|
Neurotoxicity, |
|
|
|
|
Any |
20.9 |
21.1 |
19.4 |
|
Grade 3 and 4 |
4 |
4.1 |
3.2 |
|
Grade 5‡ |
0.9 |
0.5 |
3.2 |
|
Median Time to onset of neurotoxicity, days |
4 |
4 |
4 |
|
Seizure |
14.2 |
15.5 |
6.5 |
|
Grade 3 and 4 |
|
|
|
|
Infection |
|
|
|
|
Grade 3 and 4 |
14.7 |
16.0 |
6.4 |
|
Fever |
88.0 |
87.6 |
90.3 |
|
Hypotension |
|
|
|
|
Grade 3 and 4 |
40.9 |
43.3 |
25.8 |
|
Hypoxemia |
|
|
|
|
Grade 3 and 4 |
21.8 |
23.2 |
12.9 |
|
Grade 5 adverse events |
1.3 |
1.0 |
3.2 |
|
Received tocilizumab |
74.2 |
76.8 |
58.1 |
|
Median time to first tocilizumab, |
2 |
2 |
4 |
|
Received corticosteroids |
79 |
74 |
5 |
|
Median time to first |
4 |
4 |
4 |
Conclusion
This study demonstrated encouraging and durable efficacy with CD19-/CD22-CAR T-cell therapy in children with R/R B-ALL. The outcomes were promising in patients with isolated and combined extramedullary relapse, supporting its potential as a therapeutic option for this patient population. A longer follow-up period, combined with deeper analysis afforded by next-generation sequencing technologies, may provide further insights.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

