All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Combination therapy in patients with Philadelphia chromosome-negative B-ALL
Acute lymphoblastic leukemia (ALL) is a type of hematologic cancer of immature lymphocytes in the bone marrow, which most commonly affects children but also occurs in adults.1 The prognosis is poor for older patients with Philadelphia (Ph)-negative B-cell ALL (B-ALL), with a median survival of 5–10 months, and treatment remains a challenge in this population as there is currently no standard of care.2
Novel therapies, such as blinatumomab and inotuzumab ozogamicin, have significantly improved outcomes in Ph-negative relapsed/refractory (R/R) B-ALL. In addition, venetoclax has shown promising activity for both T-cell ALL (T-ALL) and B-ALL in pre-clinical trials. There is growing evidence to suggest that combining these novel therapies with low-intensity chemotherapy, such as mini-hyper-CVD, could be more effective and safer than traditional chemotherapy for patients with R/R Ph-negative B-ALL.1
Below, we summarize three poster presentations that were presented at the European Hematology Association (EHA) 2022 Congress, which shared efficacy and safety data of combination and sequential therapies for B-ALL, including mini-hyper-CVD plus inotuzumab ozogamicin with or without blinatumomab in older adults with newly diagnosed B-ALL,2 mini-hyper-CVD plus venetoclax in patients with Ph-negative B-ALL,3 and hyper-CVAD plus blinatumomab with or without inotuzumab ozogamicin in adults with newly diagnosed Ph-negative B-ALL.4
An updated phase II trial of mini-hyper-CVD plus inotuzumab ozogamicin with or without blinatumomab in older adults with newly diagnosed B-ALL2
Study design
Patients with newly diagnosed Ph-negative B-ALL were included in the study if they were aged ≥60 years, had a performance status of 0–3, and adequate organ function.
Patients 1–49 were administered the following treatment regimen:
- Mini-hyper-CVD for ≤8 cycles, with inotuzumab ozogamicin for the first 4 cycles.
- Inotuzumab ozogamicin was given at a dose of 1.3–1.8 mg/m2 on Day 3 of Cycle 1 and 0.8–1.3 mg/m2 on Day 3 of Cycles 2–4.
- Maintenance included POMP (6-mercaptopurine + vincristine + methotrexate + prednisone) chemotherapy for ≤3 years.
Patients 50–80 were given an amended treatment regimen, as follows:
- Inotuzumab ozogamicin was administered in fractionated doses for the first 4 cycles of mini‑hyper‑CVD (0.6 mg/m2 on Day 2 and 0.3 mg/m2 on Day 8 of Cycle 1; 0.3 mg/m2 on Day 2 and 8 of Cycles 2–4), followed by 4 cycles of blinatumomab.
- Maintenance included 12 cycles of POMP and 4 cycles of blinatumomab.
Results
Baseline characteristics
Baseline characteristics of all patients included in this study are summarized in Table 1.
Table 1. Baseline characteristics of patients who received mini-hyper-CVD + inotuzumab ozogamicin ± blinatumomab*
|
CNS, central nervous system; HeH, high hyperdiploidy; Ho-Tr, low hypodiploidy/near-triploid; IM/ND, information missing/not done ; Misc, miscellaneous; Ph, Philadelphia chromosome; t, translocation; WBC, white blood cell. |
|
|
Characteristic, % (unless otherwise stated) |
N = 80 |
|---|---|
|
Median age (range), years |
68 (60–87) |
|
Age ≥70 years |
38 |
|
Performance status ≥2 |
13 |
|
Median WBC (range), ×109/L |
3.1(0.3-11.0) |
|
Karyotype |
|
|
Diploid |
32 |
|
HeH |
6 |
|
Ho-Tr |
15 |
|
Tetraploidy |
4 |
|
Complex |
4 |
|
t(4;11) |
1 |
|
Misc |
19 |
|
IM/ND |
19 |
|
CNS disease at diagnosis |
5 |
|
Median CD19 (range) |
99.5 (26–100) |
|
Median CD22 (range) |
96.9 (27–100) |
|
CD20 expression ≥20% |
60 |
|
Ph-like ALL |
19 |
|
TP53 mutation |
39 |
Efficacy and safety
As shown in Figure 1, the overall response rate (ORR) was 99%, minimal residual disease (MRD) negativity was achieved in 94% of patients, and no early deaths were reported.
Figure 1. Response rates in patients with Ph-negative B-ALL treated with mini-hyper-CVD + inotuzumab ozogamicin ± blinatumomab*
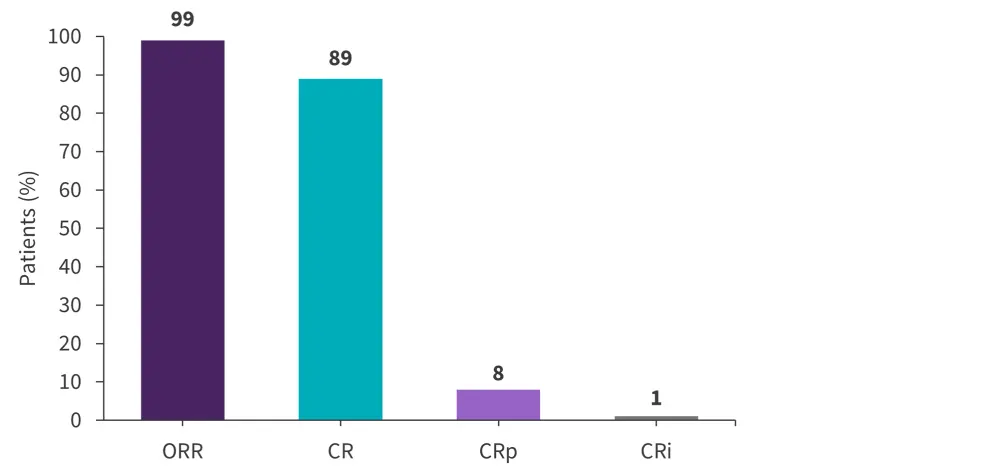
B-ALL, B-cell acute lymphoblastic leukemia; CR, complete remission; CRi, CR with incomplete count recovery; CRp, CR with incomplete platelet recovery; ORR, overall response rate; Ph, Philadelphia chromosome.
*Data from Haddad.2
Of the 79 patients who achieved remission prior to and after treatment, 33 were undergoing ongoing treatment or observation, 31 died in remission from various causes, 11 relapsed without hematopoietic stem cell transplant (HSCT), and four went on to receive HSCT while in remission. Myelodysplastic syndromes/acute myeloid leukemia developed in nine patients (seven of whom had a TP53 mutation).5
The 5-year remission and overall survival (OS) rates were 76% and 47%, respectively, and the OS outcomes were better for patients aged 60–69 years compared with patients ≥70 years, as well as for patients without poor cytogenetics compared with those with. In addition, deaths in remission were more frequent in patients aged ≥70 years compared with those aged 60–69 years.5
A phase II study of mini-hyper-CVD plus venetoclax in patients with Ph-negative ALL3
Study design
Patients were included if they were ≥18 years of age with R/R Ph-negative B-ALL or T‑ALL/lymphoblastic lymphoma or <60 years of age and unfit for intensive chemotherapy. Alternatively, patients ≥60 years with previously treated B-ALL or T-ALL who had received 1–2 courses of any frontline chemotherapy were included. Additional inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of ≤3 and adequate organ function (total bilirubin ≤3.0 mg/dL and creatinine clearance ≥ 30mL/min).
The primary endpoint was ORR (complete remission [CR] + CR with incomplete recovery) after two cycles. Secondary endpoints included MRD negativity after one cycle, duration of response, OS, and safety.
Treatment regimen
- Mini-hyper-CVD alternating with methotrexate and cytarabine was administered for ≥8 cycles.
- Venetoclax at 400 mg/day was given on Days 1–14 of Cycle 1 and Days 1–7 of Cycles 2–8.
- Rituximab (if the patient had CD20+ B-ALL) and prophylactic intrathecal chemotherapy were provided in 8 doses for the first 4 cycles.
- During consolidation, patients with T-ALL received an additional 2 cycles of nelarabine (650 mg/m2 daily on Days 1–5) and peg-asparaginase (1,500 IU/m2; capped at 3,750 IU; on Day 5) without venetoclax, and during maintenance a further 2 cycles of nelarabine + peg‑asparaginase were provided.
- Responding patients received vincristine and prednisone maintenance with venetoclax daily on Days 1–14 of each 28-day cycle for ≥2 years.
Results
Baseline characteristics
Baseline characteristics of all patients are summarized in Table 2.
Table 2. Baseline characteristics of patients who received mini-hyper-CVD + venetoclax*
|
B-ALL, B-cell acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia; T-LL, T-cell lymphoblastic lymphoma; WBC, white blood cell |
|
|
Characteristic, % (unless otherwise stated) |
N = 20 |
|---|---|
|
Median age, years (range) |
45 (20–70) |
|
Performance status ≥2 |
25 |
|
Salvage status |
|
|
Salvage 1 |
50 |
|
Salvage 2+ |
50 |
|
Prior therapies |
|
|
Prior stem cell transplant |
55 |
|
Median no. of prior therapies (range) |
1 (1–5) |
|
Prior targeted therapy in B-ALL (inotuzumab or blinatumomab) |
87 |
|
Diagnosis |
|
|
B-ALL |
75 |
|
T-ALL |
20 |
|
T-LL |
5 |
|
Median WBC (range), ×109/L |
3.9 (0.9–19.5) |
|
Median bone marrow blasts (range) |
62 (0–87) |
|
Karyotype |
|
|
Diploid |
33 |
|
Complex |
16 |
|
t(4;11) |
10 |
|
TP53 mutated |
31 |
Efficacy and safety
The median follow-up was 15 months and the median number of cycles received was 2 (range, 1–6). Of the 19 evaluable patients, an overall response was achieved by 63% (Figure 2). Overall, MRD negativity was achieved in 50% of patients.
Figure 2. Response rates in patients with Ph-negative R/R ALL treated with mini-hyper-CVD plus venetoclax*
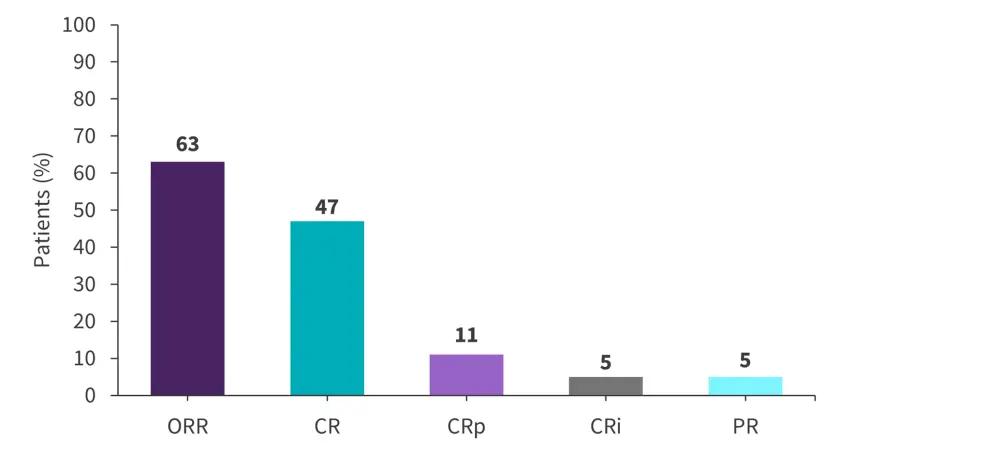
ALL, acute lymphoblastic leukemia; CR, complete remission; CRi, CR with incomplete count recovery; CRp, CR with incomplete platelet recovery; ORR, overall response rate; Ph, Philadelphia chromosome; PR, partial response; R/R, relapsed/refractory.
*Data from Senapati.3
Among the 12 responders, four patients proceeded to HSCT; the remaining eight patients who did not undergo HSCT later relapsed and died. The median OS and relapse-free survival was 7.1 months and 6.2 months, respectively.
Although four patients experienced a Grade 3 AE, no Grade 4/5 adverse events (AEs) were reported.6 The most common AEs of any grade, with two incidences observed of each, were aspartate transaminase/alanine aminotransferase elevation, back pain, and hyperbilirubinemia.
In responding patients, the median time to neutrophil and platelet recovery in Cycle 1 was 18 days and 27 days, respectively.
Hyper-CVAD plus blinatumomab with or without inotuzumab ozogamicin in adults with newly diagnosed Ph-negative B-ALL4
Study design
Patients aged ≥14 years with newly diagnosed Ph-negative B-ALL were included in the study if they were eligible for extensive chemotherapy, had an ECOG performance status ≤3, adequate organ function (bilirubin, ≤2 mg/dL; creatinine ≤2 mg/dL), and no significant central nervous system pathology.
Treatment regimen
- Alternating cycles of hyper-CVAD and high-dose methotrexate + cytarabine were administered for ≥4 cycles, with intrathecal chemotherapy given in 8 doses, followed by 4 cycles of blinatumomab.
- Maintenance therapy consisted of alternate cycles of POMP (Cycles 1–3, 5–7, 9–11, and 13–15) and blinatumomab (Cycles 4, 8, and 12).
- Patients with high-risk disease features started blinatumomab after 2 cycles of hyper-CVAD.
- For patient #39 onwards, inotuzumab ozogamicin was added at a dose of 0.3 mg/m2 on Days 1 and 8 during the 2 cycles of methotrexate + cytarabine and 2 cycles of blinatumomab.
- Patients with CD20+ disease (≥1% cells) received eight doses of ofatumumab (2,000 mg) or rituximab (375 mg/m2).
Results
Baseline characteristics
Baseline characteristics of all patients are summarized in Table 3.
Table 3. Baseline characteristics for the patients with B-ALL treated with hyper-CVAD + blinatumomab ± inotuzumab ozogamicin*
|
CD, cluster of differentiation; CNS, central nervous system; CRLF2, cytokine receptor like factor 2; INO, inotuzumab ozogamicin; KMT2A, lysine methyltransferase 2A; WBC, white blood cell count. |
|||
|
Characteristic, % (unless otherwise stated) |
Overall |
Hyper-CVAD + blinatumomab |
Hyper-CVAD + blinatumomab + INO |
|---|---|---|---|
|
Median age (range), years |
34 (17–59) |
37 (17–59) |
24 (18–47) |
|
Performance status |
|||
|
0–1 |
81 |
79 |
85 |
|
2 |
19 |
21 |
15 |
|
Median WBC (range), ×109/L |
4.1 (0.5–553) |
3.12 (0.5–360.9) |
8.1 (1.2–553) |
|
CNS involvement at diagnosis |
10 |
11 |
10 |
|
CD20 ≥20% |
55 |
52 |
63 |
|
CD19 ≥50% |
98 |
97 |
100 |
|
Karyotype |
|
|
|
|
Diploid |
31 |
29 |
35 |
|
Low hypodiploidy |
14 |
16 |
10 |
|
Complex |
7 |
8 |
5 |
|
High hypodiploidy |
7 |
8 |
5 |
|
KMT2A rearrangement |
9 |
8 |
10 |
|
Others |
33 |
32 |
35 |
|
CRLF2+ by flow cytometry |
19 |
19 |
19 |
|
TP53 mutation |
26 |
27 |
25 |
|
JAK2+ |
8 |
5 |
13 |
Efficacy and safety
Overall, all patients achieved CR at any time point, and 80% achieved CR after the first cycle. In addition, MRD negativity was achieved by 95% of patients at any time, and in 76% after induction (Figure 3).
Figure 3. Response rates in patients with Ph-negative B-ALL treated with hyper-CVAD + blinatumomab ± inotuzumab ozogamicin*
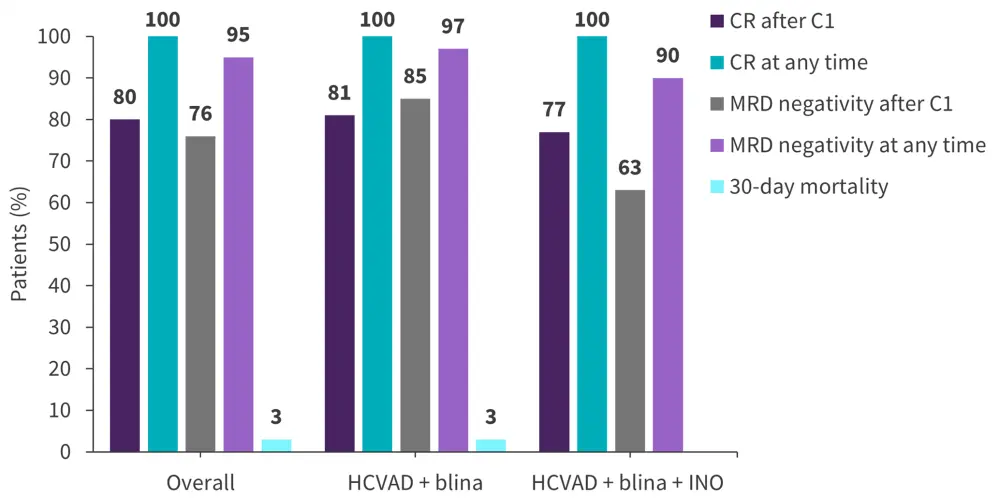
C, Cycle; CR, complete remission; HCVAD, hyper-CVAD; INO, inotuzumab ozogamicin; MRD, measurable residual disease.
Data from Short.4
Overall, 13 patients underwent HSCT in first remission, five patients did not undergo HSCT and relapsed, two patients died in CR, and 18 patients remained in CR without HSCT. The 3-year CR duration and OS rate was 84% and 85%, respectively.
One patient discontinued blinatumomab due to Grade 2 neurotoxicity; however, no patients discontinued inotuzumab ozogamicin.
Conclusion
The data summarized here confirms the potential of combination therapies in patients with Ph‑negative ALL, including the use of mini-hyper-CVD plus inotuzumab ozogamicin with or without blinatumomab in older adults with newly diagnosed B-ALL, mini-hyper-CVD combined with venetoclax in B-ALL and T-ALL, or hyper-CVAD with sequential blinatumomab with or without inotuzumab ozogamicin in Ph‑negative B‑ALL. All combination approaches were well-tolerated and safe, and could therefore offer new treatment options for patients with R/R Ph-negative B-ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

