All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Combining ofatumumab and hyper-CVAD therapy for CD20-positive, Ph-negative B-cell ALL
CD20 expression on lymphoblasts at the time of diagnosis has been found to be associated with a poor prognosis in patients with Philadelphia chromosome (Ph)-negative B-cell acute lymphoblastic leukemia (ALL). Previous experience with combining an anti-CD20 monoclonal antibody, rituximab, with a chemotherapy regimen of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) has led to improved complete remission (CR) duration and better overall survival (OS) rates.
ALL Hub Steering Committee member Elias Jabbour and colleagues investigated the efficacy and safety of another anti-CD20 monoclonal antibody, ofatumumab, combined with hyper-CVAD in patients with newly-diagnosed, Ph-negative B-cell ALL or lymphoblastic lymphoma in a single-arm, phase 2 study (NCT01363128), and reported their results in The Lancet Haematology.1
Ofatumumab targets a specific juxtamembrane small-loop extracellular epitope on CD20 and has been associated with better cytotoxicity in vitro compared with rituximab. It has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of relapsed/refractory chronic lymphoblastic leukemia,2 along with a very recent approval for relapsing multiple sclerosis,3 however, there is no evidence to date on its use in treating B-cell ALL.
Study design1
- Single-arm, open-label, single-center phase II trial, performed at the MD Anderson Cancer Center, Houston, US
- Eligibility criteria:
- Patients with newly diagnosed, Ph-negative, CD20+ (defined as expression on ≥ 1% of lymphoblasts confirmed by bone marrow assessment) B-cell ALL or B-cell lymphoblastic lymphoma, who were treatment-naïve or received one course of induction chemotherapy;
- Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and
- Adequate renal, hepatic, and cardiac function.
The number of patients included was 69 (B-cell ALL, n = 67; B-cell lymphoblastic lymphoma, n = 2). Median age was 41 years (range 32–50), with 48% of patients aged between 18 and 39 years. Patient characteristics are summarized in Table 1.
Table 1. Baseline characteristics1
|
ALL, acute lymphoblastic leukemia; CNS, central nervous system; CR, complete remission; Ph-like, Philadelphia chromosome-like; RNA, ribonucleic acid; TN, treatment-naïve. * These patients were in CR at baseline |
|
|
Characteristics |
Patients, n (%) |
|---|---|
|
CNS involvement |
2 (3) |
|
Previous treatment |
|
|
TN One cycle of therapy* |
65 (94) 4 (6) |
|
CD20 positivity |
|
|
1–19% of cells ≥ 20% of cells Positive, unspecified |
23 (33) 43 (62) 3 (4) |
|
Ph-like ALL by RNA sequencing, n/N (%) |
9/33 (27) |
|
Cytogenetics (n = 68) |
|
|
Normal karyotype High hyperdiploidy Low-hypodiploidy or near triploidy t(1;19)(q23;p13)–TCF3-PBX1 Complex karyotype (≥ 5 abnormalities) Others Insufficient metaphases |
23 (34) 7 (10) 4 (6) 4 (6) 2 (3) 20 (29) 8 (12) |
Methods
Primary outcomes included event-free survival, OS, and overall response rate (CR and CR with incomplete platelet recovery). The secondary outcome was safety, and measurable residual disease (MRD) was assessed as an exploratory endpoint.
Treatment procedure is detailed in Figure 1.
Figure 1. Study treatment1
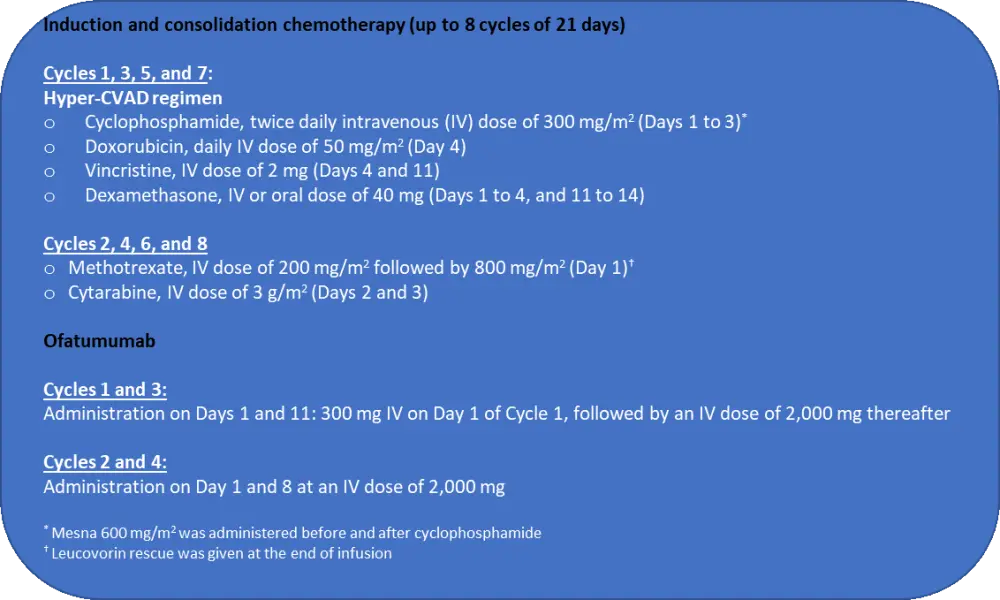
Following induction, patients received 30 courses of POMP maintenance therapy with:
- 6-mercaptopurine, oral dose of 50 mg three times daily,
- Vincristine, intravenous dose of 2 mg every 28 days,
- Methotrexate, oral dose of 20 mg/m2 weekly, and
- Prednisone, oral dose of 200 mg daily.
During Cycles 6 and 18, asparaginase 2,000 IU/m² was administered on Day 1. Patients who did not receive hematopoietic stem cell transplantation (HSCT) were given a total of 12 doses of ofatumumab. Methotrexate and cytarabine were used for central nervous system prophylaxis.
Treatment response was evaluated following each cycle until CR, and every three to four cycles once CR was achieved.
Results
During a median follow-up of 22 months (range, 26–53), 46 patients (67%) were alive, 37 patients (54%) were alive and in first CR, and median OS was not reached. Twenty-one patients (30%) had morphological relapse, of those, 12 (30%) died due to disease progression. Median time from CR to relapse was 15 months (range, 10–25). Other outcomes are summarized in Table 2.
Table 2. Results of efficacy analysis1
|
CI, confidence interval; MRD, measurable residual disease; NR, not reached; OS, overall survival. |
|
|
Outcome |
Patients |
|---|---|
|
Overall response (n = 65), n (%) |
|
|
After one cycle After two cycles |
62 (95) 64 (98) |
|
MRD negativity, n/N (%) |
|
|
After one cycle Overall |
40/63 (63) 63/68 (93) |
|
Median time to MRD negativity, months (range) |
0.7 (0.4–4.8) |
|
Event-free survival |
|
|
Median, months (range) Estimated 4-year event-free survival, % (range) |
51 (44–NR) 59 (48–73) |
|
OS |
|
|
Median, months (range) 4- year OS, % (range) |
NR (65–NR) 68 (58–81) |
|
Post-hoc subgroup analyses |
|
|
4-year event-free survival, % (95% CI) |
|
|
Patients aged < 40 years Patients aged ≥ 40 years Patients aged ≥ 55 years |
69 (54–87) 51 (37–71) 38 (16–87) |
|
4-year OS, % (95% CI) |
|
|
Patients aged < 40 years Patients aged ≥ 40 years Patients aged ≥ 55 years |
74 (60–91) 63 (49–81) 56 (31–100) |
Post-hoc subgroup analyses demonstrated that JAK2 mutation was associated with poor event-free survival (HR, 4.71; 95% CI, 1.50–14.82; p = 0.0081) and OS (HR, 8.06; 95% CI, 2.23–29.16; p = 0.0014). The occurrence of MRD negativity following induction chemotherapy was significantly lower in patients with Ph-like ALL compared to those without (22% and 75%, respectively, p = 0.02).
Safety
Study treatment was tolerable with most side effects being Grade 1–2, and half of the patients (51%) completed the planned eight cycles. During induction period, median times to absolute neutrophil and platelet count recovery were 19 days (range, 17–22) and 16 days (range, 14–17), respectively. During the consolidation period, corresponding median recovery times were 21 days (range, 20–25), and 22 days (range, 19–26).
One patient died during induction chemotherapy due to septic shock, while ten (14%) patients died in CR due to sepsis (n = 2), cardiac arrest (n = 1), complications associated with HSCT (n = 5), and therapy-related acute myeloid leukemia. Sepsis-related deaths occurred in patients aged > 40 years and were considered to be unrelated to study treatment.
There was no treatment discontinuation due to treatment-related toxicity, however, 34 patients (49%) had their number of chemotherapy cycles reduced due to tolerability issues.
Grade ≥ 3 adverse events (AEs) were more common in older patients than those aged 18–39 years. The most common non-hematological AEs are presented in Table 3.
Table 3. The most common Grade ≥ 3 non-hematological adverse events1
|
AE, adverse event. |
|||
|
AE |
Grade 3 |
Grade 4 |
Grade 5 |
|---|---|---|---|
|
Infections, n/N (%) Induction chemotherapy Consolidation chemotherapy |
32/65 (49) 41/68 (60) |
3/65 (5) 12/68 (18) |
1/65 (2) 2/68 (3) |
|
Hyperglycemia, n (%) |
33 (48) |
1 (1) |
– |
|
Hypokalemia |
31 (45) |
4 (6) |
– |
|
Increased aminotransferase levels |
25 (36) |
1 (1) |
– |
|
Hypophosphatemia |
21 (30) |
– |
– |
|
Hypocalcemia |
16 (23) |
4 (6) |
– |
|
Hyperbilirubinemia |
13 (19) |
3 (4) |
– |
|
Hypoalbuminemia |
11 (16) |
– |
– |
|
Hyponatremia |
11 (16) |
– |
– |
|
Ofatumumab infusion reaction |
5 (7) |
– |
– |
Other common Grade 3 AEs included nausea or vomiting, mucositis, headache, fatigue, diarrhea, peripheral neuropathy, edema, abdominal pain, acute kidney injury and syncope.
Conclusion
The combination of ofatumumab and hyper-CVAD regimen was associated with durable remissions, high rates of 4-year event-free survival, and OS in adult patients with Ph-negative B-cell ALL. The treatment benefits for patients aged between 18–39 years were similar to those reported with pediatric patients. The safety profile was comparable to that of hyper-CVAD, alone or combined with rituximab. This study enrolled patients with low level of CD20 positivity (1–19%) who would have been excluded from CD20-targeted therapies in previous trials. In the light of the study data, ofatumumab combined with hyper-CVAD can be considered a safe and effective frontline therapeutic option for patients with Ph-negative CD20+ B-cell ALL.
Limitations of this study included a study design (single arm and single center) that did not allow a direct comparison with rituximab in terms of safety and efficacy, a limited generalizability of outcomes, and the lack of statistical power to test differences in outcomes between subgroups.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

