All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Daratumumab for relapsed/refractory T-cell ALL or LL: Results from DELPHINUS study
Pediatric T-cell acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LL) are aggressive, chemoresistant malignancies with poor prognosis and survival outcomes. Approximately 15–20% of pediatric patients with ALL or LL will be refractory or relapse after front-line treatment.1 Preliminary studies suggested that daratumumab (DARA) combined with conventional chemotherapy would be effective against T-cell ALL/LL.
DARA, a human immunoglobulin G1k monoclonal antibody that binds CD38, is approved for the treatment of multiple myeloma and has shown preclinical efficacy in ALL models. CD38 is a type II transmembrane glycoprotein that is highly expressed on myeloma cells and blasts in patients with T-cell ALL at diagnosis and relapse; therefore, it is a promising target for immunotherapy.2,3 The phase II DELPHINUS study (NCT03384654) examined the safety and efficacy of DARA in addition to standard chemotherapy in pediatric/young adult patients with relapsed/refractory T-cell ALL and T-cell LL; results were presented by Laura E. Hogan at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting.3 Below, we summarize the key findings.
Study design
This is an open-label, non-randomized phase II clinical trial evaluating the efficacy and safety of DARA in addition to vincristine, prednisone, L-asparaginase, and doxorubicin (VPLD) in pediatric and young adult patients (1–30 years of age) with T-cell ALL/LL who are refractory to one prior induction/consolidation regimen or in first relapse. Patients received age/risk-adjusted intrathecal therapy and response was measured by local bone marrow morphology at the end of each cycle.
Dosing schedule
DARA (16 mg/kg IV once a day on Days 1, 8, 15, and 22) was given in Cycles 1–2.
- Cycle 1: DARA in combination with VPLD (backbone)
- Vincristine: 1.5 mg/m2 (maximum: 2.0 mg/m2) IV once a day on Days 1, 8, 15, and 22
- Prednisone: 40.0 mg/m2 PO divided twice a day on Days 1–28
- PEG-asparaginase: 2,500 U/m2 IM/IV once a day on Days 2 and 16
- Doxorubicin: 60.0 mg/m2 IV once on Day 1
- Cycle 2: DARA in combination with
- Methotrexate: 5.0 g/m2 IV once on Day 2
- Cyclophosphamide: 1.0 g/m2 IV once on Day 15
- Cytarabine: 75.0 mg/m2 IV/SC once a day on Days 16–19 and Days 23–26
- 6-mercaptopurine: 60.0 mg/m2 PO once a day on Days 15–28
Endpoints
- Primary endpoint was complete response (CR) rate in pediatric patients with T-cell ALL at the end of Cycle 1.
- Secondary endpoints included:
- Overall response rate: CR or CR with incomplete hematological recovery at any time before subsequent therapy or hematopoietic stem cell transplant
- Minimal residual disease negativity
- Event-free survival and overall survival
- Safety and tolerability
- Pharmacokinetics of DARA
Results
Patient baseline characteristics
A total of 22 pediatric and five young adult patients with T-cell ALL and ten patients with T-cell LL were enrolled in this clinical trial.
- T-cell ALL (pediatric): 22 patients completed Cycle 1, 18 completed Cycle 2, and six received DARA continuation.
- T-cell ALL (young adult): five patients completed Cycle 1, three completed Cycle 2, and one received DARA continuation
- T-cell LL: ten patients completed Cycle 1, six completed Cycle 2, and no patients received DARA continuation.
- All T-cell ALL patients expressed CD38 at baseline; further details on baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics*
|
ALL, acute lymphoblastic leukemia; LL, lymphoblastic lymphoma. ‡Time from the initial diagnosis to the first dose. |
|||
|
Characteristics |
Pediatric T-cell ALL |
Young adult T-cell ALL |
T-cell LL |
|---|---|---|---|
|
Median age (range), years |
10.0 (2–17) |
23.0 (18–25) |
14.5 (5–22) |
|
Sex, % |
|
|
|
|
Female |
41.7 |
0 |
10.0 |
|
Male |
58.3 |
100.0 |
90.0 |
|
Median time‡ (range), |
2.5 (0.5–6.1) |
0.6 (0.1–5.6) |
0.8 (0.5–6.0) |
Efficacy
- Overall, 41.7% of pediatric patients with T-cell ALL achieved CR at the end of Cycle 1.
- Overall response rate was 83.3%, 60%, and 40% in pediatric patients with T-cell ALL, young adult patients with T-cell ALL, and patients with T-cell LL, respectively (Figure 1).
- In total, 41.7% of pediatric patients with T-cell ALL and 20.0% of young adult patients with T-cell ALL achieved minimal residual disease negativity at any time during treatment.
- The median follow-up was 31.3 months, 25.4 months, and 16.4 months for pediatric patients with T-cell ALL, young adult patients with T-cell ALL, and patients with T-cell LL, respectively.
- Median event-free survival was 8.3 months, 8.0 months, and 2.9 months in pediatric patients with T-cell ALL, young adult patients with T-cell ALL, and patients with T-cell LL, respectively.
- Overall survival was 9.5 months in the pediatric patients with T-cell ALL, 13.6 months in the young adult patients with T-cell ALL, and 4.2 months in the patients with T-cell LL.
Figure 1. ORR in patients who received 16 mg/kg IV DARA once a day*
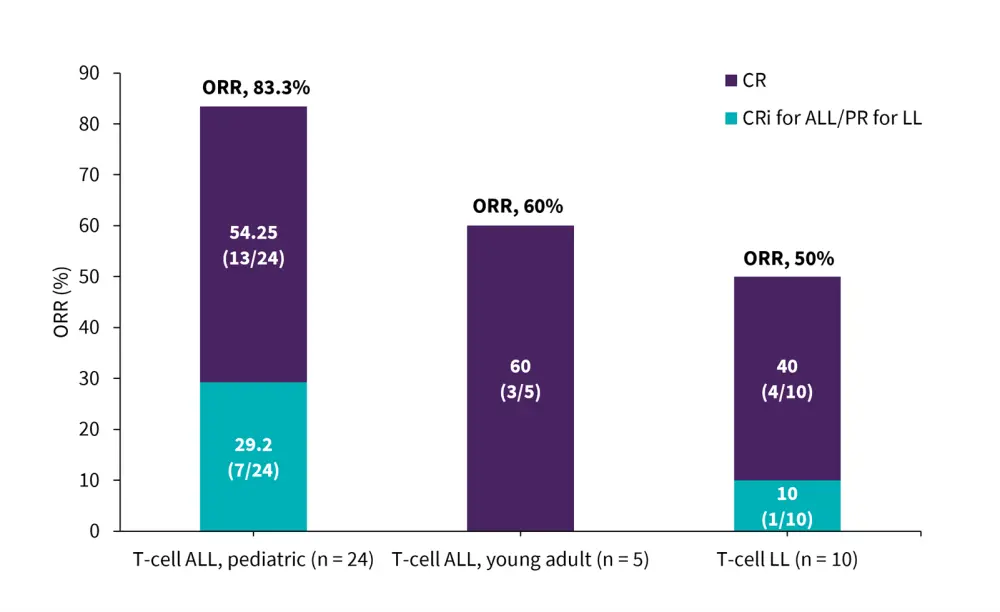
ALL, acute lymphoblastic leukemia; CR, complete response; Cri, CR with incomplete hematologic recovery; IV, intravenous; LL, lymphoblastic lymphoma; ORR, overall response rate; PR, partial response.
*Adapted from Hogan.3
Safety
- Adverse events (AEs) with DARA were consistent with those commonly seen with VPLD backbone therapy alone.
- The most common AEs included anemia, thrombocytopenia, neutropenia, and febrile neutropenia (Table 2).
- All pediatric patients with T-cell ALL had a Grade 3/4 treatment-emergent adverse event, 12 patients (50.0%) experienced a Grade 3/4 infection, and three patients (12.5%) experienced Grade 3/4 sepsis.
- No pediatric patients with T-cell ALL discontinued DARA due to AEs.
- One patient (4.2%) died of brain edema and hepatic failure attributed to study treatment (asparaginase allergy) but unrelated to DARA.
Table 2. TEAEs in pediatric patients with T-cell ALL*
|
AE, adverse event; TEAE, treatment-emergent adverse event. |
|
|
Grade 3/4 TEAE† (≥20% of patients), % |
Patients (n = 24) |
|---|---|
|
Anemia |
66.7 |
|
Thrombocytopenia |
62.5 |
|
Neutropenia |
50.0 |
|
Febrile neutropenia |
45.8 |
|
Leukopenia |
37.5 |
|
Increased alanine aminotransferase |
25.0 |
|
Hypokalemia |
25.0 |
Overall, 16 (66.7%) pediatric patients with T-cell ALL, four (80.0%) young adult patients with T-cell ALL, and eight (80.0%) patients with T-cell LL, experienced ≥1 infusion-related reaction of any grade. The most common infusion-related reactions in pediatric patients with T-cell ALL were abdominal pain, pyrexia, vomiting, and nausea (Table 3).
Table 3. Infusion-related reactions in pediatric/young adult patients with T-cell ALL*
|
Infusion-related reaction†, % |
T-cell ALL, pediatric |
T-cell ALL, young |
T-cell LL |
|---|---|---|---|
|
Any grade occurring in ≥10% of patients |
|||
|
Abdominal pain |
25.0 |
0 |
10.0 |
|
Pyrexia |
16.7 |
0 |
10.0 |
|
Vomiting |
12.5 |
0 |
10.0 |
|
Nausea |
12.5 |
0 |
0 |
|
Cough |
12.5 |
0 |
40.0 |
|
Urticaria |
12.5 |
0 |
0 |
|
Rash |
4.2 |
20.0 |
0 |
|
Dyspnea |
4.2 |
0 |
30.0 |
|
Pruritus |
0 |
20.0 |
10.0 |
|
Rhinitis |
0 |
20.0 |
0 |
|
Increased gamma |
0 |
20.0 |
0 |
|
Musculoskeletal chest pain |
0 |
20.0 |
0 |
|
Grade 3/4 occurring in any patients |
|||
|
Leukopenia |
4.2 |
0 |
0 |
|
Abdominal pain |
4.2 |
0 |
0 |
|
Thrombocytopenia |
0 |
0 |
10.0 |
|
Bronchospasm |
0 |
0 |
10.0 |
|
ALL, acute lymphoblastic leukemia; LL, lymphoblastic lymphoma. |
|||
Pharmacokinetics
For pediatric patients with T-cell ALL, the mean serum concentration of DARA was 361 µg/mL on Day 22 of Cycle 2; this was higher than identified effective concentrations in adult patients with multiple myeloma,4 suggesting a DARA dose of 16 mg/kg IV once a day is sufficient.
Conclusion
In this phase II trial, DARA showed initial activity in combination with VPLD in pediatric and young adult patients with relapsed/refractory T-cell ALL or LL. The addition of DARA resulted in improved response rates, compared with VPLD backbone therapy alone, and had a manageable safety profile.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

