All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Efficacy and safety of obe-cel in R/R adult B-ALL: Phase II FELIX study
CD19-directed chimeric antigen receptor (CAR) T-cell therapies have revolutionized the treatment landscape for relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL).1 Obecabtagene autoleucel (obe-cel) is a novel autologous CD19 CAR T-cell therapy with a fast off-rate CD19 binding domain designed to improve persistence and mitigate safety concerns. Based on its clinical activity in R/R pediatric and adult B-ALL, as well as other B-cell malignancies within the phase I ALLCAR19 study (NCT02935257), it is now being investigated in adult patients with R/R B-ALL (FELIX study; NCT04404660).1
Below, we summarize key efficacy and safety data from the FELIX study, presented by Roddie at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting.1
Study design
This is an open-label, multi-center, single-arm phase Ib/II study, which included adult patients aged ≥18 years with R/R B-ALL and ≥5% bone marrow (BM) blasts. The treatment schedule involved a split dose infusion of obe-cel on Day 1 and Day 10 up to a target dose of 410 x 106 CAR T cells. To maximize therapeutic benefit, the dosing schedule was adjusted based on tumor burden prior to lymphodepletion and severity of toxicities after first infusion (Figure 1).
The primary endpoint was overall remission rate, defined as a complete response (CR) or CR with incomplete blood count recovery (CRi) by central assessment. Secondary endpoints included duration of response, event-free survival, overall survival, minimal residual disease (MRD) negativity rate, and safety.
Figure 1. Study design*
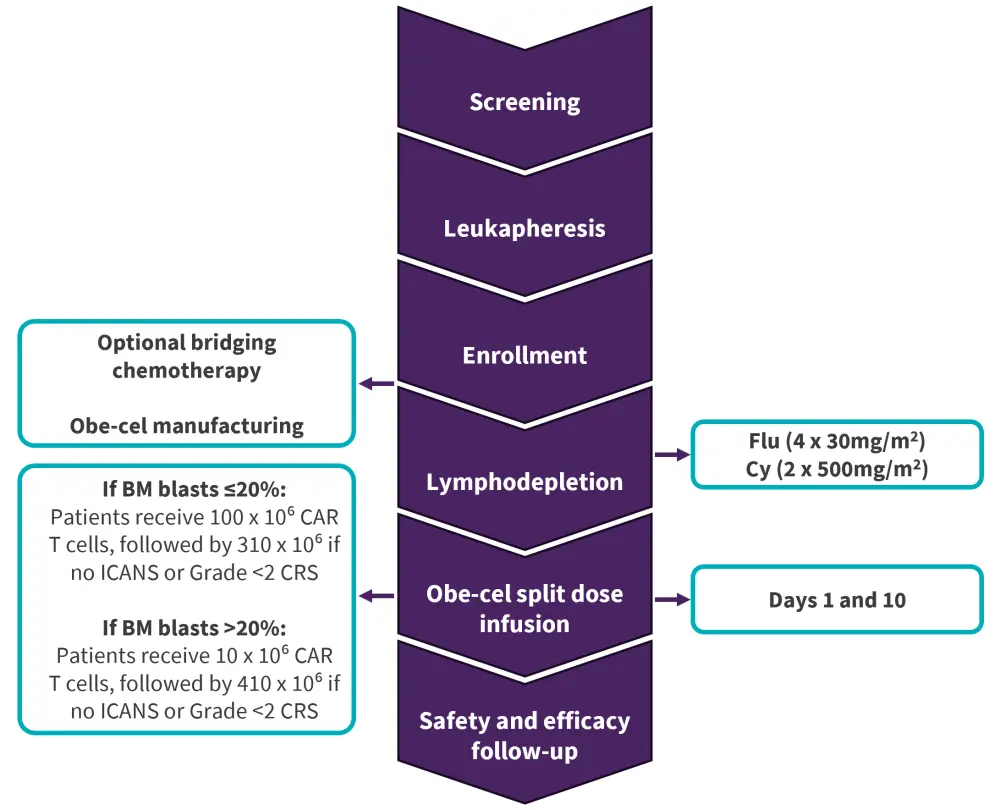
BM, bone marrow; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; Cy, cyclophosphamide; DLT, dose-limiting toxicity; Flu, fludarabine; ICANS, immune effector cell-associated neurotoxicity; obe-cel, obecabtagene autoleucel.
*Adapted from Roddie, et al.1
Results
Of the 112 patients enrolled, 84% were infused with obe-cel, 94% of whom received both infusions. Patients were heavily pretreated and presented with high disease burden; baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
BM, bone marrow; EMD, extramedullary disease; obe-cel, obecabtagene autoleucel; SCT, stem cell transplantation. |
|
|
Characteristic, % (unless otherwise stated) |
Obe-cel infused |
|---|---|
|
Median age at diagnosis (range), years |
50 (20–81) |
|
Sex |
|
|
Male |
50 |
|
Female |
50 |
|
Philadelphia-chromosome positive |
26.6 |
|
Prior lines of therapies, median (range), n |
2 (1–6) |
|
≥3 prior lines |
30.9 |
|
Refractory to last prior line of therapy |
53.2 |
|
Prior therapies |
|
|
SCT |
38.3 |
|
Blinatumomab |
35.1 |
|
Inotuzumab |
31.9 |
|
Blinatumomab + inotuzumab |
16.0 |
|
Median BM blasts at screening (range) |
49.5 (6–100) |
|
Median BM blasts at lymphodepletion (range) |
41.1 (0–100) |
|
EMD at lymphodepletion |
19.1 |
Efficacy and cellular kinetics
At the median follow-up of 9.5 months, 76% of patients achieved a CR/CRi. MRD negativity at a sensitivity level of 10-4 by flow cytometry was achieved by 97% of responders, and durable responses were observed in 61% of responders. The median duration of response was 14.1 months.
Consistent with the ALLCAR19 study, obe-cel demonstrated early expansion with ongoing CAR T persistence.
Safety
Among all patients infused, the incidence of any-grade cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) was 75.5% and 25.5%, respectively; with Grade ≥3 CRS or ICANS observed in 3.2% and 7.4% of patients, respectively. The rate of CRS and ICANS was relative to disease burden, higher in patients with high (>20% BM blasts) versus low (≤20% BM blasts) tumor burden at lymphodepletion. For CRS, tocilizumab was given to 56% of patients, steroids in 17%, and vasopressor was only required in 3%.
Overall, any grade and Grade ≥3 treatment-emergent adverse events occurred in 98.9% and 78.7% patients, respectively. The most common Grade ≥3 treatment-emergent adverse events were neutropenia, thrombocytopenia, febrile neutropenia, and anemia (Figure 2). Only one treatment-related death was reported.
Figure 2. Most common Grade ≥3 TEAEs*
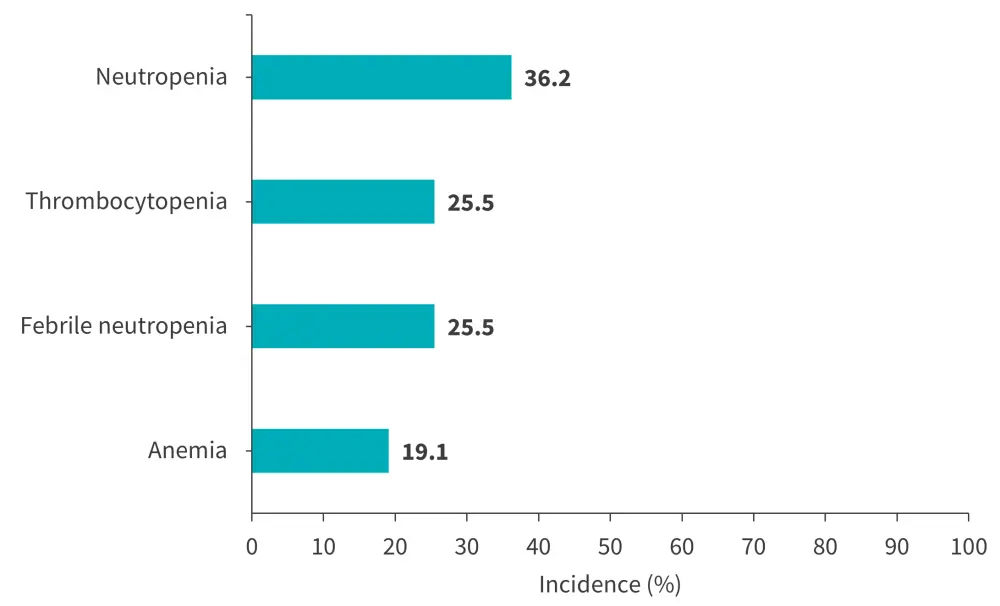
TEAE, treatment-emergent adverse event.
*Data from Roddie, et al.1
Presenter’s conclusion
In the phase II FELIX study, obe-cel demonstrated deep and durable responses, and a manageable safety profile in heavily pretreated adult patients with R/R B-ALL. High CR/CRi rates, high numbers of MRD-negative remissions, ongoing persistence, and very low rates of Grade ≥3 CRS and ICANS were reported, thus obe-cel represents a promising option for this patient subset.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

