All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
First-in human-trial of revumenib in patients with KMT2A-rearranged or NPM1-mutated leukemia
Introduction
Lysine methyltransferase 2A rearrangements (KMT2Ar) and nucleophosmin 1 gene (NPM1) mutations arise in 80% of pediatric patients with acute lymphoblastic leukemia and ~30% of adult patients with acute myeloid leukemia. Revumenib, a potent selective oral inhibitor of the menin–KMT2A interaction, prevents the assembly of oncogenic KMT2A wild-type or fusion complexes on chromatin and has demonstrated significant anti-leukemic activity in preclinical KMT2Ar or NPM1-mutated leukemia models.
Currently, there are no targeted therapies specifically approved for acute leukemia with KTM2A or NPM1 mutations. Below, we summarize an article by Issa et al.1 published in Nature on a first-in-human trial investigating the efficacy and safety of revumenib in patients with KMT2A-rearranged or NPM1-mutated relapsed/refractory (R/R) acute leukemia.
Study design
This is a phase I, multicenter, open-label dose-escalation study conducted across nine sites in the United States. Included patients were
- aged 1–79;
- had received a median of four previous lines of therapy; and
- had R/R acute leukemia with KTM2A rearrangements or mutated NPM1.
The study consisted of two parallel dose escalation cohorts of patients, with revumenib administered orally every 12 hours in continuous 28-day cycles (Figure 1). Primary endpoints included safety, maximum tolerated dose, recommended phase II dose, and characterization of the pharmacokinetic parameters of revumenib based on CYP3Ai. Exploratory endpoints included assessment of anti-leukemic activity in patients treated with KMT2Ar or mutated NPM.
Figure 1. Trial design*
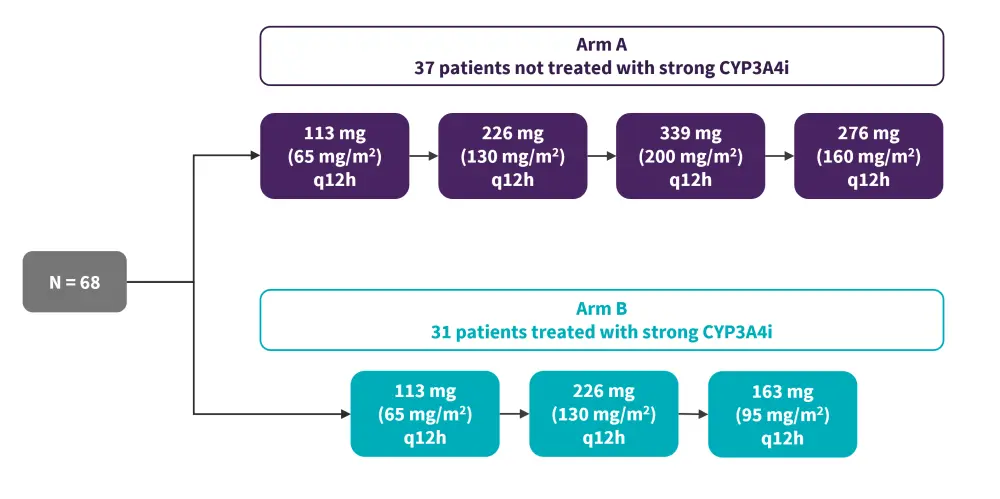
CYP3A4i, cytochrome P450 3A4 inhibitor; KMT2A, Lysine methyltransferase 2A; KMT2Ar, KMT2A rearranged; NPM1, nucleophosmin 1; q12h, every 12 hours; R/R, relapsed/refractory.
*Adapted from Issa, et al.1
Results
At the data cutoff (March 31, 2022), a total of 68 patients were treated, 82% of whom had R/R acute myeloid leukemia, 16% had acute lymphoblastic leukemia, and 2% had mixed-phenotype acute leukemia. Additionally, 46% had KMT2Ar, 21% had mutated NPM1, and 12% had neither mutation. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; HSCT, hematopoietic stem cell therapy; KMT2A, histone-lysine N-methyltransferase 2A; MPAL, mixed-phenotype acute leukemia; NPM1, nucleophosmin 1; RAS, renin-angiotensin-system; TP53, tumor protein 53. |
|
|
Characteristic, % (unless otherwise stated) |
Whole cohort (N = 68) |
|---|---|
|
Median age, years |
42.5 |
|
Male |
38 |
|
Female |
62 |
|
Median bone marrow blasts |
59.5 |
|
Acute leukemia type |
|
|
AML |
82 |
|
Therapy-related AML |
10 |
|
ALL |
16 |
|
MPAL |
2 |
|
Genotype |
|
|
KMT2Ar |
68 |
|
t(9;11)(p21;q23) |
15 |
|
t(4;11)(q21;p23) |
9 |
|
t(11;19)(q23;p13.1) |
7 |
|
t(11;19)(q23;p13.3) |
6 |
|
t(6;11)(q27;q23) |
4 |
|
t(11;17)(q23;q12) |
3 |
|
Other 11q23 translocations |
24 |
|
Mutated NPM1 |
21 |
|
KMT2Ar and NPM1 wild type |
12 |
|
Co-occurring mutations |
|
|
FLT3 |
25 |
|
RAS |
29 |
|
TP53 |
10 |
|
Previous therapies |
|
|
Median number of prior therapies (range), n |
4 (1–12) |
|
Venetoclax |
60 |
|
Allogeneic HSCT |
46 |
Safety
Among the treated cohort, 67 patients (99%) experienced any-grade treatment-emergent adverse events (TEAE), and 53 (78%) had any-grade treatment-related adverse event (TRAE).
The most common any-grade TEAEs were prolongation of the QT interval, nausea, vomiting and febrile neutropenia; the incidence of the most common TEAE’s occurring in ≥20% of patients are reported in Figure 2.
Figure 2. Most common TEAEs (occurring in ≥20% of patients)*
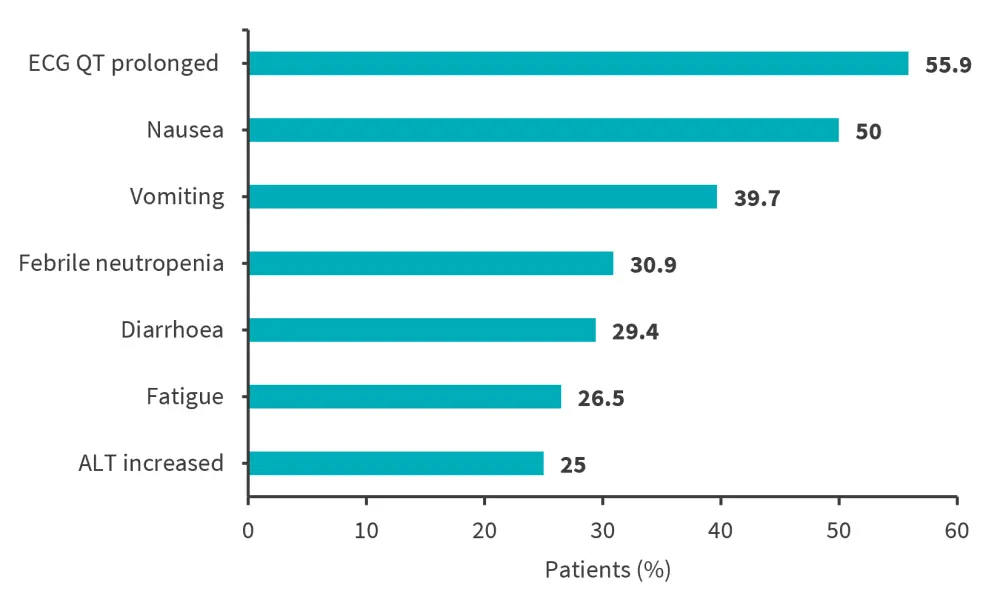
ALT, alanine transaminase; ECG, electrocardiogram; TEAE, treatment-emergent adverse event.
*Adapted from Issa, et al.1
- The most common Grade 3 treatment-related adverse event was the prolongation of the QT interval, occurring in nine patients; all were able to continue treatment at the next lower dose level.
- No treatment-related adverse events led to death or discontinuation of revumenib.
- Grade 2 differentiation syndrome was reported in 11 patients and all cases were resolved following corticosteroid treatment.
Pharmacokinetics and efficacy
- Doses of 226 mg and 276 mg every 12 hours in Arm A met the safety, tolerability, and pharmacokinetic prespecified criteria for the recommended phase II dose.
- Doses of 113 mg and 163 mg every 12 hours met the same prespecified criteria in Arm B.
- The overall response rate in efficacy-evaluable patients was 53% (n = 60).
- The rate of complete remission or complete remission with partial hematologic recovery was 30%, 78% of whom achieved undetectable minimal residual disease.
Conclusion
This study highlights that menin inhibition with revumenib monotherapy has encouraging anti-leukemic activity and safety in both children and adults with relapsed/refractory KMT2Ar or NPM1 mutated leukemia. Revumenib induced complete remissions and was associated with low Grade 3 or higher treatment-emergent adverse events.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

