All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Hypersensitivity reactions to asparaginase: Incidence, identification, management, and overcoming challenges
Do you know... When administered every 48 hours, what is the recommended dose of asparaginase erwinia chrysanthemi (recombinant)-rywn?
Asparaginase is a key component of treatment regimens for acute lymphocytic leukemia (ALL), with asparaginases either derived from E.coli, or Erwinia.1 There are native or pegylated forms of asparaginase available, with five asparaginase-based products approved by the U.S. Food and Drug Administration (FDA). Of these, two native asparaginase products are no longer marketed.1
Asparagine is needed for cellular growth, and is synthesized by healthy cells via the asparagine synthase enzyme.2,3 Leukemia cells rely on exogenous asparagine for growth, as they are unable to synthesize asparagine. Administration of asparaginase leads to depletion of exogenous asparagine, ultimately leading to leukemia cell death.2,3
As asparaginase treatments utilize foreign enzymes, there is a risk of immune responses in patients. Therefore, ensuring availability of multiple asparaginase therapy options with different immunogenic profiles is key for treatment success.2
Use of asparaginase in ALL
There are multiple asparaginase products used regularly in the treatment of ALL in the US; availability of asparaginase can vary in other countries, with an additional recombinant E.coli derived L-asparaginase product used in Europe (Table 1).4
Pegylation of asparaginase involves attachment of polyethylene glycol (PEG), which can decrease the immunogenicity and improve duration of action of the asparaginase.2 Immune reactions, including hypersensitivity reactions (HSRs), are a common adverse event related to asparginase treatment; therefore, availability of pegylated asparaginase formulations with decreased immunogenicity is important.2
In clinical trials and systemic reviews, pegaspargase, a pegylated asparaginase, was found to decrease the incidence of antibodies compared with native E. coli asparaginase, with a similar safety profile between the two products.2 Calaspargase pegol-mknl is a newer, long-acting asparaginase product that is being used for the treatment of ALL and has shown improved sustained activity compared with pegaspargase.2
Erwinia asparaginase is indicated in patients with hypersensitivity to E.coli-derived asparaginase and is generally well-tolerated, with most patients achieving clinically effective asparaginase activity.2
Table 1. Currently used asparaginase products in the treatment of ALL*
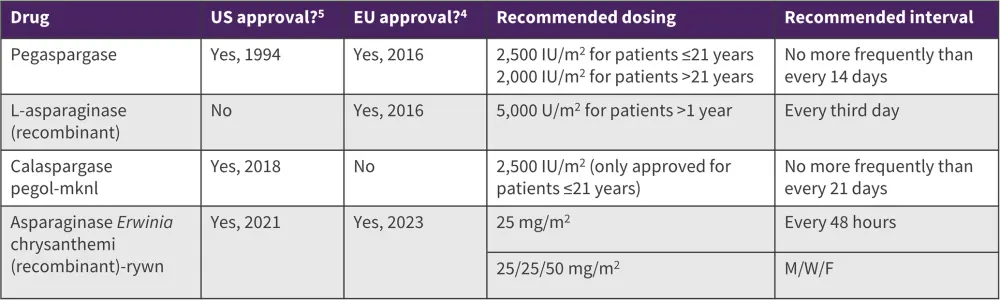
ALL, acute lymphocytic leukemia; M/W/F, Monday, Wednesday, Friday.
*Data from European Medicines Agency4 and U.S Food and Drug Administration.5
Question 1 / 1
Which of the following asparaginase products is the most recently approved by the FDA?
A
Pegaspargase
B
Calaspargase pegol-mknl
C
Erwinia asparaginase
D
Asparaginase Erwinia chrysanthemi (recombinant)-rywn
Incidence of hypersensitivity reactions
HSRs are immunogenic reactions related to asparaginase treatment and indicate the development of anti-asparaginase antibodies, reducing the effectiveness of the therapy (Figure 1). HSRs are common adverse events that often occur after the first dose of asparaginase.2
Approximately 30% of patients will experience an HSR after E. coli asparaginase treatment.6 The risk of HSR varies depending on the asparaginase product, with repeated administration carrying a higher risk.2 Other factors that influence hypersensitivity include timing of treatment, genetics, and use of mitigation techniques.3 A number of premedication mitigation techniques are used, such as corticosteroids and antihistamines; however, these have shown mixed results.3 After switching to an asparaginase with a different immunogenic profile, only ~25% of patients will experience additional HSRs, allowing ≥90% of patients to complete an asparaginase treatment regimen.2
Figure 1. The primary immune response following asparaginase infusion*
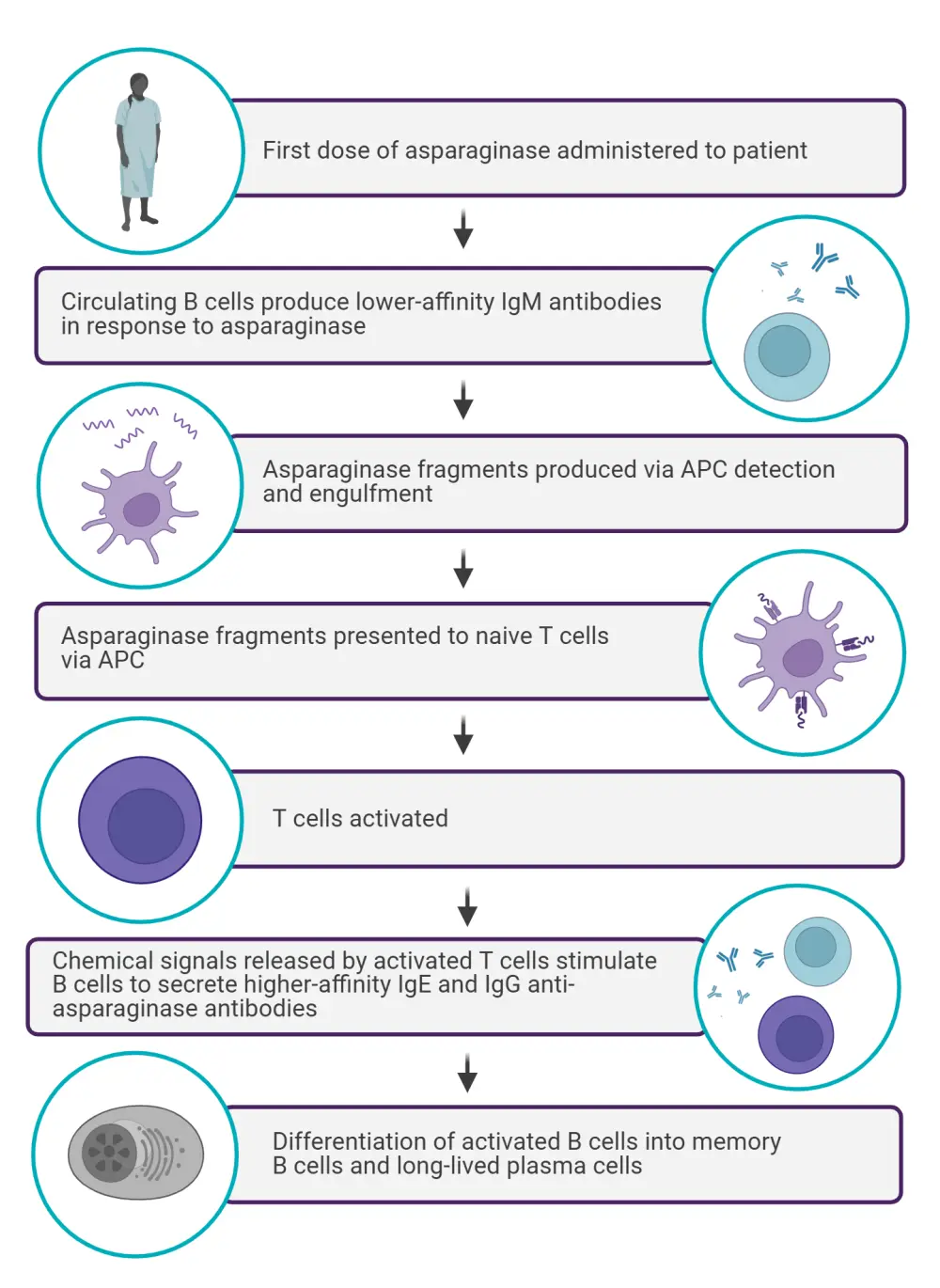
APC, antigen-presenting cell.
*Data from Burke et al.2 Created with Biorender.com.
Question 1 / 1
What is the approximate incidence of HSR in patients who switch to an asparaginase with a different immunogenic profile following an initial HSR?
A
25%
B
35%
C
50%
D
65%
Identification of HSRs
A range of symptoms can be attributed to HSRs, from mild injection site reactions to severe systemic reactions, which can include anaphylaxis.7 Severity of HSR can be graded based on the Common Terminology Criteria for Adverse Events (CTCAE) classification (Table 2), and this criteria can be used to assist in identifying HSRs in clinical practice. However, challenges remain, as there may be difficulties in determining whether a mild, Stage 1 reaction is a true HSR. In these instances, monitoring serum asparaginase level can help to inform treatment decisions and management of the patient.7
Table 2. CTCAE classification of allergic reactions*
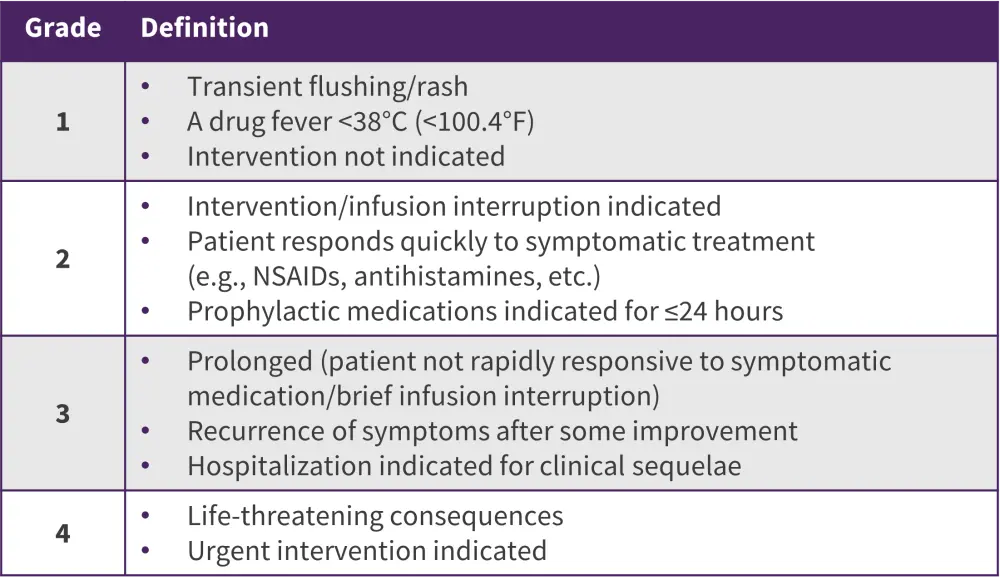
CTCAE, Common Terminology Criteria for Adverse Events; NSAID, non-steroidal anti-inflammatory drug.
*Data from Van der Sluis et al.7
Management of HSRs
Therapeutic drug monitoring (TDM) allows for asparaginase activity to be measured regularly and can be used to determine which patients may be at risk of silent inactivation, where antibody inactivation of asparaginase occurs without HSR symptoms.1,2 A serum asparaginase activity (SAA) level ≥0.1 IU/mL is the defined target for effective asparaginase depletion, although some studies have suggested a lower threshold of 0.02 IU/mL with pegaspargase.1 A study investigated the use of TDM to monitor efficacy maintenance following treatment switching in pediatric patients.6 During induction, median trough asparaginase activity varied depending on the product used, therefore demonstrating the utility of TDM in assessing whether efficacy targets will be reached after treatment switching.6
TDM can also assist in differentiating between antibody-mediated HSRs and infusion reactions, which can inform treatment decisions.6 With any severe reactions, infusions should be stopped, and further treatment will be determined by the type of reaction; if an HSR is likely, switching to an Erwinia-derived asparaginase is recommended, for example see suggested evidence-based recommendations from the Children’s Oncology Group (Figure 2)1. Where Erwinia-derived asparaginase is unavailable, patients with HSRs can undergo drug desensitization with E. coli-derived asparaginases.2 Recommendations can vary between clinics and countries, although consensus guidelines recommend switching asparaginase therapy in patients who experience HSRs, antibody-mediated reactions, or silent inactivation.2
Figure 2. Treatment paradigm in patients experiencing a reaction during PEG-asparaginase infusion*
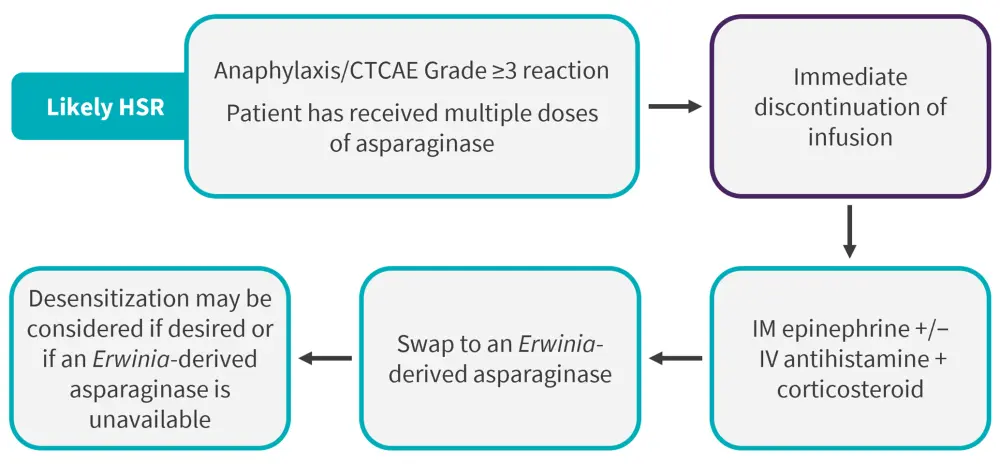
CTCAE, Common Terminology Criteria for Adverse Events; HSR, hypersensitivity reaction; IM, intramuscular; IV, intravenous; PEG, polyethylene glycol.
*Data from Sandley et al.1
Management of HSRs and general toxicity differs between pediatric and adult patients, as adolescent and adult patients may experience higher rates of toxicity when treated with pediatric-focused regimens.1 The CALGB 10403 and COG AALL0230 studies have shown similar rates of severe HSRs between pediatric and adult patients (14.6% vs 10%, respectively). However, a higher occurrence of other toxicities in older patients, such as thrombosis, hyperglycemia, and pancreatitis, was observed in these studies. Dose capping or reduced doses of pegaspargase (≤1,000 IU/m2) are often used in adults to mitigate these risks. An expert consensus recommended using premedication in adults with ALL receiving asparaginase therapy and differentiating between HSRs and other reactions. Utilizing these strategies is key in reducing the potential toxicities and HSRs following asparaginase treatment, particularly in adult patients.1
Safety and efficacy of recombinant Erwinia asparaginase in clinical trials
A phase II/III study, AALL1931 (NCT04145531), investigated asparaginase Erwinia chrysanthemi (recombinant)-rywn in 167 pediatric and adult patients with ALL and lymphoblastic lymphoma who had experienced hypersensitivity to E coli derived asparaginase.8 The primary endpoint was the proportion of patients with the last 72-hour nadir serum asparaginase activity (NSAA) level ≥0.1 IU/mL.8
The patients were grouped into three cohorts: 1a, 25 mg/m2 on Monday, Wednesday, and Friday (MWF); 1b, 37.5 mg/m2 MWF; 1c, 25/25/50 mg/m2 MWF. The proportion of patients achieving NSAA level ≥0.1 IU/mL was similar across all cohorts in the last 48 hours but was higher in Cohorts 1b and 1c compared with Cohort 1a in the last 72 hours (Figure 3)8.
Figure 3. Proportion of patients achieving NSAA level ≥0.1 IU/mL*
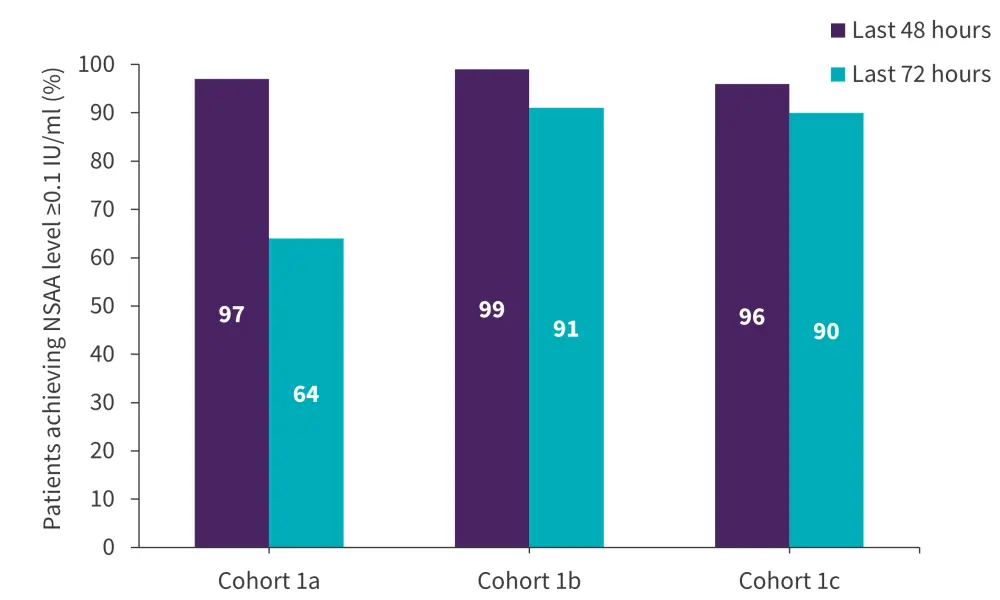
NSAA, nadir serum asparaginase activity.
*Adapted from Maese et al.8
In total, 74.3% of patients experienced treatment-related adverse events (TEAEs); the most common non-hematologic TEAEs were vomiting and nausea (Figure 4). Allergic reactions related to treatment occurred in 9.6% patients, with 1.8% experiencing an anaphylactic reaction. None of the patients experienced TEAEs leading to death.8
Figure 4. A Most common non-hematologic TEAEs and B most common TEAEs of special interest*
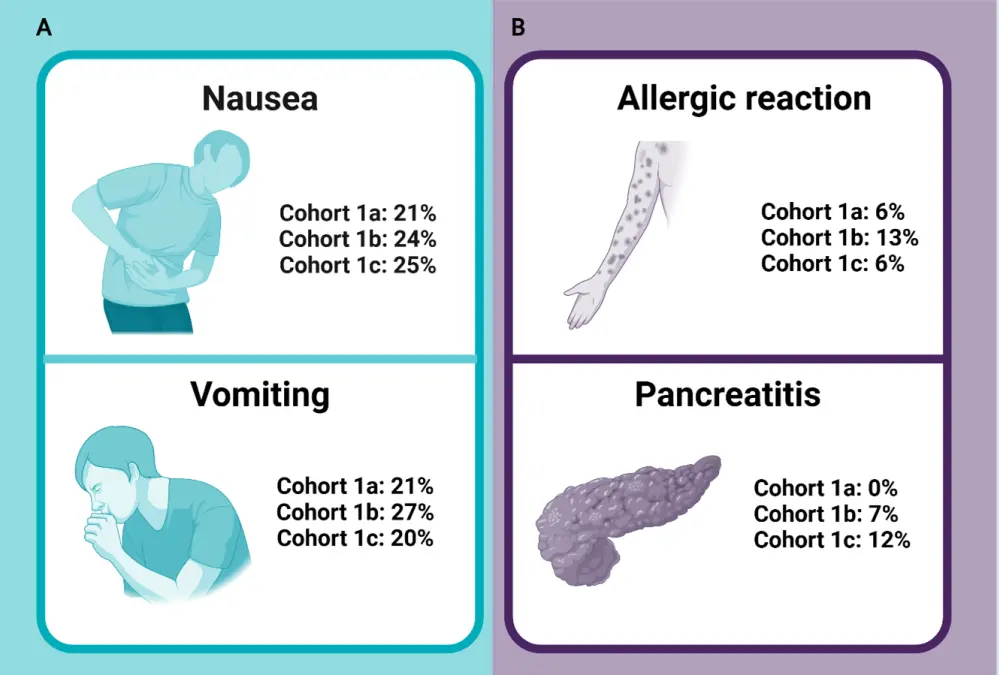
TEAE, treatment-emergent adverse event.
*Data from Maese et al.8 Created using Biorender.com.
This study highlights that asparaginase Erwinia chrysanthemi (recombinant)-rywn at a dose of 25/25/50 mg/m2 MWF is efficacious and tolerable in patients with ALL and leads to NSAA level ≥0.1 IU/mL in most patients at 48 and 72 hours. Lower serum levels of asparaginase may increase relapse risk, highlighting the importance of this treatment for patients with ALL, especially when there are shortages of other therapies. Further studies are needed to evaluate the impact of Erwinia chrysanthemi on patient outcomes.8
Following on from positive clinical trial results, Erwinia chrysanthemi (recombinant)-rywn was first approved by the FDA in 2021 in adult and pediatric patients 1 month or older with and hypersensitivity to E. coli-derived asparaginase, as part of a multi-agent chemotherapeutic regimen at a dose of 25 mg/m2 intramuscularly every 48 hours.9 In 2022, an additional dosing schedule of 25 mg/m2 intramuscularly on Monday and Wednesday and 50 mg/m2 intramuscularly on Friday was approved in the same indication and patient population.10
Overcoming challenges with recombinant asparaginase
Historically, shortages of asparaginase have occurred due to the processes involved in isolating native constructs.1 This has necessitated flexibility in the formulations used in clinical practice.1 The development of recombinant formulations for both Erwinia and E.coli asparaginases, which enables efficient manufacturing processes, have overcome prior supply shortages. Moreover, recombinant formulations provide potential to further improve immunogenicity and stability, enhancing ALL treatment.
It is likely that HSRs will remain to be a challenge in asparaginase treatment for patients with ALL. However, asparaginase provides an important clinical benefit for patients with ALL, with best outcomes seen in patients who are able to complete the full course of asparaginase.1 Use of prophylactic regimens to manage toxicities, and integration of personalized approaches for each patient such as treatment switching or treatment desensitization are therefore key in improving patient outcomes. Importantly, the availability of Erwinia chrysanthemi (recombinant)-rywn provides a reliable option for treatment switching for patients experiencing HSRs with E.coli asparaginase, maximizing the number of patients able to complete their asparaginase course.1
Conclusion
Despite challenges with toxicities such as HSRs, which can result in treatment discontinuation and poor outcomes, asparaginase remains a key treatment option in patients with ALL.1 Completion of asparaginase treatment courses is essential for optimal patient outcomes, highlighting the importance of having asparaginases with alternative immunogenic profiles, such as Erwinia-derived asparaginase to mitigate HSRs.1 Reliable manufacturing of recombinant asparaginases will also help to ensure more patients are able to complete treatment courses.8 Future perspectives for asparaginase therapy in ALL include using SAA levels to individualize dosing and using pharmacogenetics to predict risk and reduce incidence of toxicities, including HSRs.11 Additional studies investigating the best HSR mitigation strategies, including optimization of strategies for both pediatric and adult patients, will further improve clinical outcomes.
This educational resource is independently supported by Jazz Pharmaceuticals. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
Your opinion matters
After reading this article, I commit to reviewing the latest data on asparaginase use for ALL, with awareness of possible adverse events including HSRs, and apply my learnings to guide my management of patients with ALL in clinical practice.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



