All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Impact of early PEG-asparaginase discontinuation in young adults with ALL
Asparaginase is an essential part of acute lymphoblastic leukemia (ALL) treatment; however, it is often discontinued early due to associated toxicities such as allergic reactions, pancreatitis, thrombosis, and hepatotoxicity.1 The introduction of pegylated (PEG)-asparaginase in pediatric ALL treatment has improved survival outcomes in both children and adults with ALL. Early PEG-asparaginase discontinuation have been found to negatively impact survival outcomes in children with ALL.2
Previous studies have indicated a higher frequency of asparaginase-related toxicities and a higher probability of early asparaginase discontinuation in adult patients compared with children.2 Currently, there is limited evidence on the correlation between early PEG- asparaginase and its effect on survival outcomes in adult patients with ALL. As such, there is a need to elucidate the prognostic implications in this patient subset in clinical settings, which can further guide treatment decisions. For example, whether to proceed with allogeneic hematopoietic cell transplantation (allo-HCT) in first complete remission (CR1), a more intensive regimen, or less intensive approaches such as combination chemotherapy.2
Below, we summarize an article by Aldoss et al., published in Blood Advances,2 examining the prevalence, risk factors, and prognostic effect of early PEG-asparaginase discontinuation in young adults with ALL.
Methods
This was a post-hoc analysis of the multicenter phase II CALGB 10403 study (NCT00558519). Overall, 176 patients were included aged 17–39 years who achieved a CR or CR with incomplete count recovery, completed remission induction with or without extended induction, consolidation, interim maintenance cycles, and initiated delayed intensification (DI) therapy whilst in remission. Patients removed from the study prior to DI or those who proceeded allo-HCT consolidation in CR1 were not included.
The study endpoints were:
- The prevalence and risk factors of early PEG-asparaginase discontinuation, defined as receiving <4 doses upon starting DI therapy.
- The impact of early PEG-discontinuation on overall survival (OS) and event-free survival (EFS) overall and by subgroup analysis of age, body mass index, measurable residual disease (MRD) status, and disease-risk.
High-risk disease was defined as the presence of high-risk cytogenetics, Philadelphia-chromosome like genotype, and a high white blood cell count; standard-risk disease was defined by the absence of these characteristics.
Results
Prevalence and risk factors of early PEG-asparaginase discontinuation
Within the patient cohort (N = 176), there was a median of five PEG-asparaginase doses given before DI. The prevalence of early PEG-asparaginase discontinuation in young adults with ALL, with less than four doses administered, was 32%. The rate of discontinuation in patients receiving one, two, and three doses was 7.4%, 13.1%, and 11.9%, respectively. In this early discontinuation subset, patients received a median of two doses. Among the remaining 68% of patients who received four or more doses of PEG-asparaginase therapy, there was a median of five doses administered.
Among all analyzed variables, only age was found to be a significant risk factor associated with early discontinuation, with a higher median age for those who received less than four doses compared with those who received at least four doses of PEG-asparaginase therapy (26 years vs 23 years; p = 0.023; Table 1). For other clinical and disease characteristics, there was no significant difference between patients receiving less than four doses compared to those receiving at least four doses.
Table 1. Selected clinical and disease characteristics in patients receiving <4 and ≥4 doses of PEG-asparaginase*
|
BMI, body max index; CRLF2, cytokine receptor like factor 2; N/A, not applicable; Ph-like, Philadelphia chromosome-like; WBC, white blood cell. |
||||
|
Characteristic, % (unless otherwise stated) |
<4 doses (n = 57) |
≥4 doses (n = 119) |
Total (N = 176) |
p value |
|---|---|---|---|---|
|
Median age (range), years |
26 (18–39) |
23 (17–38) |
24 (17–39) |
0.023 |
|
Sex |
|
|
|
0.868 |
|
Male |
64.9 |
63.0 |
63.6 |
|
|
Female |
35.1 |
37.0 |
36.4 |
|
|
PEG-asparaginase doses |
|
|
|
<0.001 |
|
Median, n |
2 |
5 |
5 |
|
|
Extended induction status |
|
|
|
0.148 |
|
Did not receive |
96.5 |
89.1 |
91.5 |
|
|
Received |
3.5 |
10.9 |
8.5 |
|
|
Median BMI, n |
26.3 |
25.7 |
26.1 |
0.215 |
|
Immunophenotype |
|
|
|
0.339 |
|
B-cell |
82.5 |
75.6 |
77.8 |
|
|
T-cell |
17.5 |
24.4 |
22.2 |
|
|
Cytogenetics |
|
|
7 |
0.086 |
|
Missing, n |
35 |
51 |
86 |
|
|
Favorable |
0 |
11.8 |
8.9 |
|
|
Intermediate |
86.4 |
83.8 |
84.4 |
|
|
Unfavorable |
13.6 |
4.4 |
6.7 |
|
|
WBC |
|
|
|
0.335 |
|
Missing, n |
0 |
3 |
3 |
|
|
≤30 |
82.5 |
75 |
77.5 |
|
|
>30 |
17.5 |
25 |
22.2 |
|
|
Ph-like |
|
|
|
1.000 |
|
Tested cases, n |
31 |
59 |
90 |
|
|
No |
69.2 |
68.3 |
68.6 |
|
|
Yes |
30.8 |
31.7 |
31.4 |
|
|
CRLF2 |
|
|
|
0.396 |
|
Tested cases, n |
31 |
59 |
90 |
|
|
No |
73.1 |
81.7 |
79.1 |
|
|
Yes |
26.9 |
18.3 |
20.9 |
|
|
Cause of death |
|
|
|
0.246 |
|
N/A† |
70.2 |
81.5 |
77.8 |
|
|
Not related to |
7.0 |
2.5 |
4.0 |
|
|
Protocol disease |
21.1 |
15.1 |
17.0 |
|
|
Protocol treatment |
1.8 |
0.8 |
1.1 |
|
|
Risk group |
|
|
|
1.000 |
|
High risk |
35.1 |
34.5 |
34.7 |
|
|
Standard risk |
64.9 |
65.5 |
65.3 |
|
Early-PEG-asparaginase discontinuation and survival outcomes
With a median follow-up of 59.6 months, the 5-year EFS and OS rates were 64.5% and 75.3%, respectively.
The 5-year OS rate was lower in patients who discontinued treatment early compared with those who did not (66.1% vs 79.6%); however, the difference was not statistically significant (p = 0.06). There was no difference found in EFS between the two groups (Figure 1).
Figure 1. Five-year OS and EFS rates for patients receiving <4 and ≥4 doses of PEG-asparaginase*
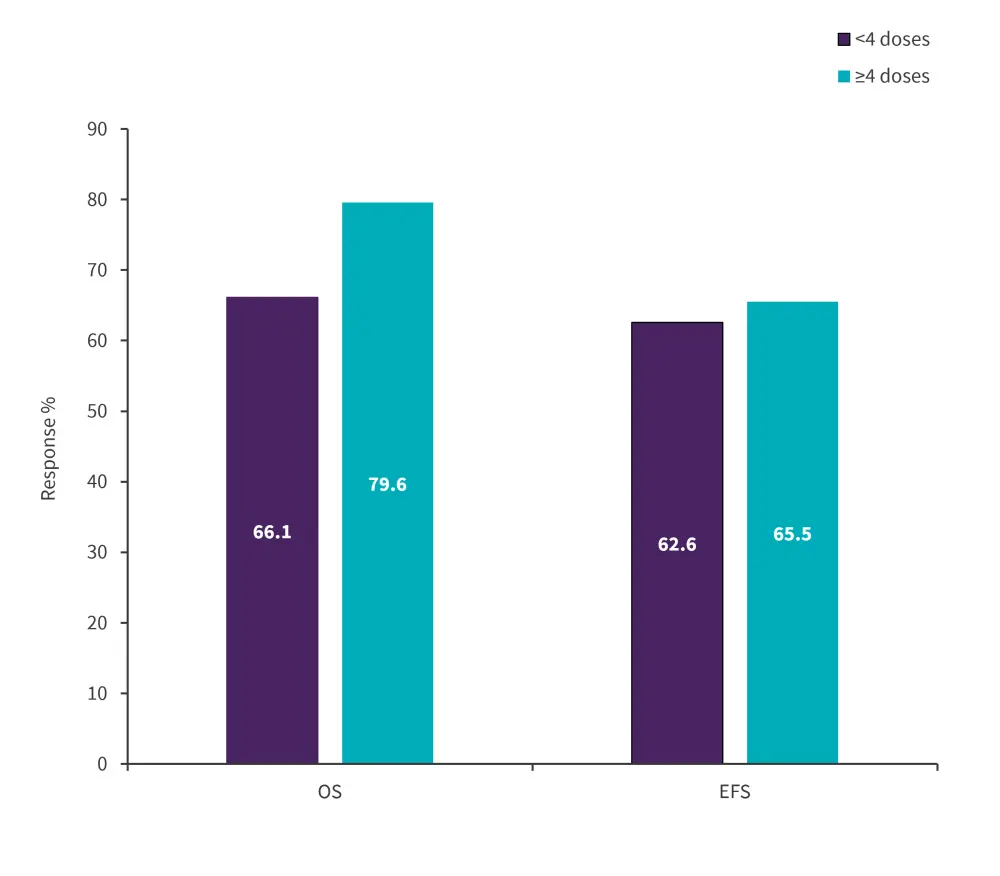
EFS, event-free survival; OS, overall survival, PEG, pegylated.
*Data from Aldoss, et al.2
The impact of early PEG-asparaginase discontinuation was found to be conflicting when comparing standard- and high-risk patients. A negative impact of early discontinuation on EFS and OS was observed in patients with standard-risk ALL, whereas there was no effect on survival outcomes in the high-risk patients (Table 2).
Table 2. Five-year survival outcomes in standard- and high-risk patients receiving PEG-asparaginase*
|
EFS, event-free survival; OS, overall survival, PEG, pegylated. |
||
|
Outcomes, % |
OS |
EFS |
|---|---|---|
|
Standard-risk patients |
|
|
|
<4 doses |
67.3 |
60.4 |
|
≥4 doses |
83 |
74.5 |
|
High-risk patients |
|
|
|
<4 doses |
63.2 |
66.3 |
|
≥4 doses |
73.2 |
48.8 |
Age, body mass index, and undetected MRD did not influence early PEG-asparaginase truncation and survival outcomes; however, there was a negative association of early discontinuation with survival in patients with detectable MRD (47% vs 78%; p = 0.06).
In a multivariate model analysis of PEG-asparaginase including dose as a continuous variable with age, cytogenetics, Ph-like status, WBC at diagnosis, body mass index, and post induction MRD, only Ph-like status predicted inferior OS and EFS.
Conclusion
This analysis demonstrates the impact of early PEG-asparaginase discontinuation to be substantial in young adults with ALL; older age was correlated with a higher risk of early truncation. Overall, early discontinuation was associated with poorer survival outcomes, a trend observed across standard-risk patients. To effectively guide treatment decisions, analysis of a larger study cohort is required to clarify the link between early PEG-asparaginase discontinuation and survival outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


