All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Inotuzumab ozogamicin for adult patients with B-ALL in CR with positive MRD: a phase II study
Detecting measurable residual disease (MRD) is a prognostic biomarker of relapse and survival in acute lymphoblastic leukemia (ALL). In the phase III INO-VATE trial, inotuzumab ozogamicin (InO) demonstrated a higher median overall survival (OS) vs standard chemotherapy (7.7 vs 6.7 months), with an MRD negativity rate of 78%.
Here, we summarize the efficacy and safety results from a phase II trial (NCT03441061) of InO in MRD positive adult patients with B-cell ALL in complete remission (CR), published by Jabbour et al.1 in Blood.
Study design1
- This study included adult patients aged ≥18 years with B-cell ALL who were in first CR (CR1) or beyond (CR2+) with positive MRD (≥1 × 10−4).
- Patients received InO at 0.6 mg/m2 on Day 1 and 0.3 mg/m2 on Day 8 of Cycle 1, followed by 0.3 mg/m2 on Days 1 and 8 of Cycles 2−6.
- The primary endpoint was relapse-free survival.
- Secondary endpoints included OS; MRD negativity rate, defined as undetectable MRD by multicolor flow cytometry in Philadelphia chromosome-negative ALL and undetectable MRD by multicolor flow cytometry and polymerase chain reaction in Philadelphia chromosome-positive ALL; and safety.
Efficacy1
- A total of 26 patients with a median age of 46 years (range, 19–70 years) were treated between November 2018 and June 2022
- Of treated patients, 73% were in CR1 and 27% were in CR2 and beyond
- Overall, 69% of patients achieved MRD-negativity after Cycle 1
- Of responders (n = 18), six patients underwent allogeneic stem cell transplantation, and five patients experienced relapse in the bone marrow (n = 3), in the bone marrow and lymph nodes (n = 1), and in the central nervous system and bone marrow (n = 1)
- There was a total of nine deaths, due to disease progression (n = 4), allogeneic stem cell transplantation complications (n = 2), septic shock (n = 1), sinusoidal obstruction syndrome (n = 1), and unknown (n = 1).
At a median follow-up of 24 months, the median relapse-free survival was 41 months in the total cohort and responders, and the median OS was not reached in the total cohort and responders; 2-year survival rates are reported in Figure 1.
Figure 1. 2-year response rates*
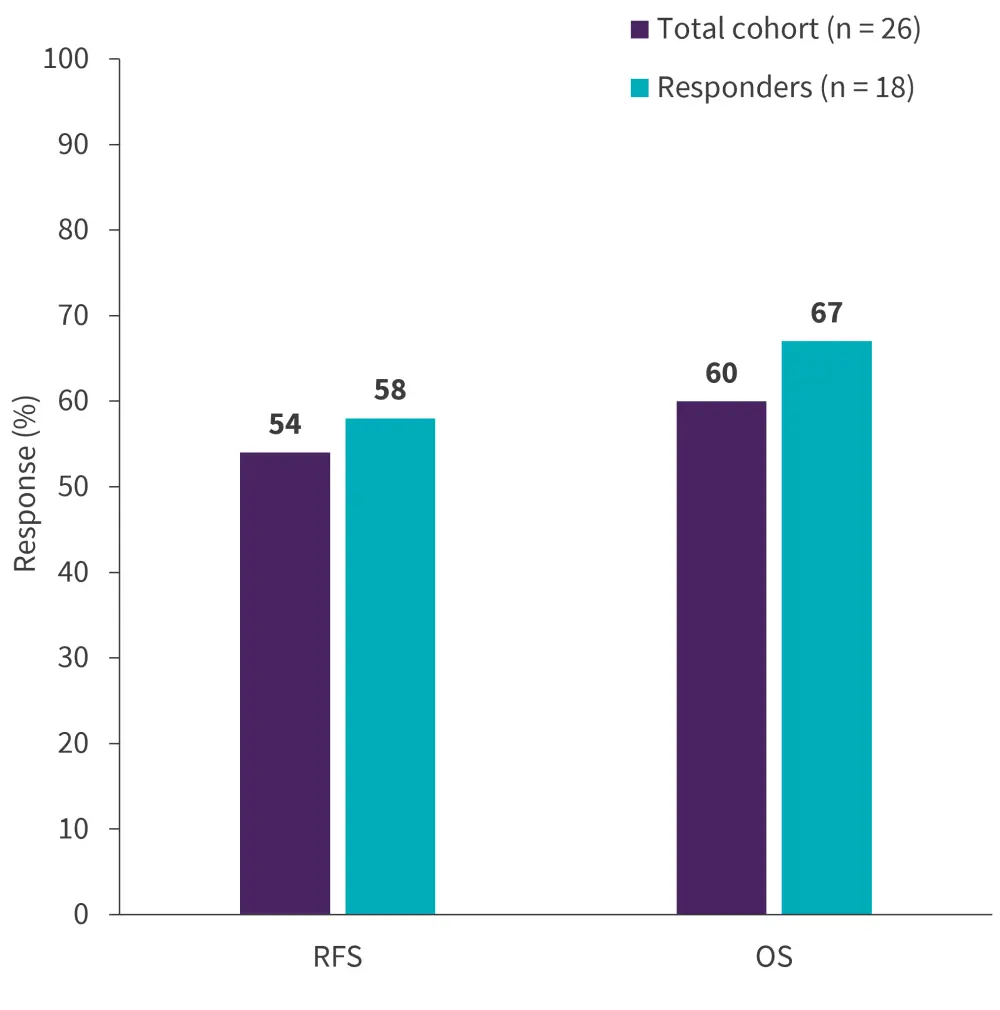
OS, overall survival; RFS, relapse-free survival
*Data from Jabbour, et al.1
Safety1
- The most common treatment-related adverse events reported were aspartate aminotransferase/alanine aminotransferase elevations and thrombocytopenia (Table 1).
- Sinusoidal obstruction syndrome was reported in two patients; one case was severe and developed 1 month after Cycle 5 and the other was a moderate case that developed during Cycle 5
Table 1. Treatment-related AEs*
|
AE, % |
Ph− negative ALL (n = 10) |
Ph+ ALL (n = 16) |
|
AE, adverse event; ALL, acute lymphoblastic leukemia; ALT/AST, alanine aminotransferase/aspartate aminotransferase; Ph, Philadelphia chromosome; SOS, sinusoidal obstruction syndrome †Grade 3 and Grade 4 both 20%. |
||
|
ALT/AST level elevation |
60 |
75 |
|
Thrombocytopenia |
70† |
63‡ |
|
Neutropenia |
50§ |
25‖ |
|
Fatigue |
20 |
13 |
|
Abdominal pain/distension |
0 |
19 |
|
Constipation |
10 |
13 |
|
Hepatic SOS |
0 |
13 |
|
Hypertension |
10 |
6 |
|
Anemia |
10 |
0 |
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


