All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Late effects of disease and treatment in survivors of adult ALL
Pediatric-based therapies have significantly improved outcomes in adult acute lymphoblastic leukemia (ALL), yielding complete remissions up to 90% and survival rates of 60–70% in younger adults. Intensive chemotherapy is associated with acute and long-term toxicities and adult patients often proceed to stem cell transplantation (SCT) due to the presence of high-risk features. Therefore, there is an increasing need to characterize comorbidities and late effects in this patient subset.1
While various systematic analyses of pediatric ALL have described comorbidity syndromes, such as central nervous system (CNS) disorders, obesity, osteonecrosis, and secondary malignancies, as well as late effects−graft-versus-host disease (GvHD), cardiovascular, pulmonary, endocrine, or musculoskeletal disorders, and secondary malignancies, there is limited data on the health status in long-term adult survivors of adult ALL.1
Below, we summarize the article published by Gokbuget et al.1 in Hematologica on the late effects, comorbidities, and general health of long-term survivors of adult ALL in German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) studies.
Study design1
This is a retrospective study of newly diagnosed adult (15–65 years) patients with ALL enrolled in six GMALL studies (NCT00198978, NCT00199004, NCT00199017, NCT00199069, NCT00199056, and NCT00198991) between 1984 and 2003 and treated with intensive, pediatric based chemotherapy according to a Berlin-Frankfurt-Munster backbone.
Responsible physicians at the participating study hospitals completed a three-part questionnaire based on the last follow-up assessments:
- Part 1: Comorbidities observed across eight organ systems (skin, lung, neurologic system, endocrine system, kidney/liver, cardiac system, gastrointestinal system, and eyes)
- Part 2: Specific comorbidity syndromes observed, including fatigue, GvHD, secondary malignancies, infections, osteonecrosis, hyperthyroidism, and hypothyroidism
- Part 3: General health condition assessment, measured by Eastern Cooperative Oncology Group Performance Status (ECOG PS).
Endpoints
The study endpoints were:
- general comorbidity status, defined as the occurrence of at least one comorbidity in nine organ categories or specific syndromes versus occurrence of no comorbidity;
- ECOG status, defined as 0−1 versus 2−4; and
- correlation of patient or disease related factors (gender, age at diagnosis, history of SCT versus no SCT, and trial cohort) with comorbidities and syndromes.
Results1
Baseline characteristics
Of the 1,413 long-term survivors of ALL enrolled in the GMALL studies, 538 were included in this analysis, with 295, 197, and 46 patients documented by the physician, practitioner, or both, respectively. A total of 584 questionnaires were eligible. The median age at diagnosis was 29 years with a median follow-up of 7 years; baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics in trials 02−04, 05, and 06−07*
|
SCT, stem cell transplantation *Data from Gokbuget, et al.1 |
||||
|
Characteristic, % (unless |
Trials 02-04 |
Trial 05 |
Trials 06-07 |
Total |
|---|---|---|---|---|
|
Median age at diagnosis (range), years |
28 (15–60) |
29 (15–64) |
30 (15–64) |
29 (15−64) |
|
≤25 |
30 |
37 |
34 |
36 |
|
26−55 |
60 |
58 |
61 |
60 |
|
>55 |
2 |
5 |
5 |
5 |
|
Sex |
|
|
|
|
|
Male |
56 |
62 |
63 |
61 |
|
Female |
44 |
38 |
37 |
39 |
|
Median age at follow-up (range), years |
46 (22–72) |
38 (20–74) |
36 (19–69) |
39 (19−74) |
|
≤25 |
1 |
4 |
17 |
10 |
|
25−55 |
73 |
76 |
72 |
74 |
|
>55 |
26 |
17 |
11 |
16 |
|
Follow-up of ≤7.5 years |
1 |
16 |
99 |
50 |
|
Median follow-up |
16.5 |
9 |
5 |
7 |
|
Follow-up from |
3−24 |
4−14 |
3−8 |
3−24 |
|
SCT |
14 |
21 |
47 |
168 |
|
Chemotherapy alone |
86 |
79 |
53 |
370 |
Late effects: Health condition and comorbidities
Overall health condition, and incidence of comorbidities/specific syndromes
Among the 522 patients evaluable for ECOG PS, an ECOG of 0 (no restrictions), 1 (slight restrictions), and 2−4 (relevant restrictions) were documented in 70%, 24%, and 6%, respectively. In total, 34% of patients did not experience any significant comorbidities and 66% had any comorbidity (n = 355). The most commonly reported comorbidities were neurological, endocrine, and skin disorders; the overall incidences of comorbidities and specific syndromes are summarized in Figure 1.
Figure 1. Overall incidence of comorbidities and specific syndromes*†
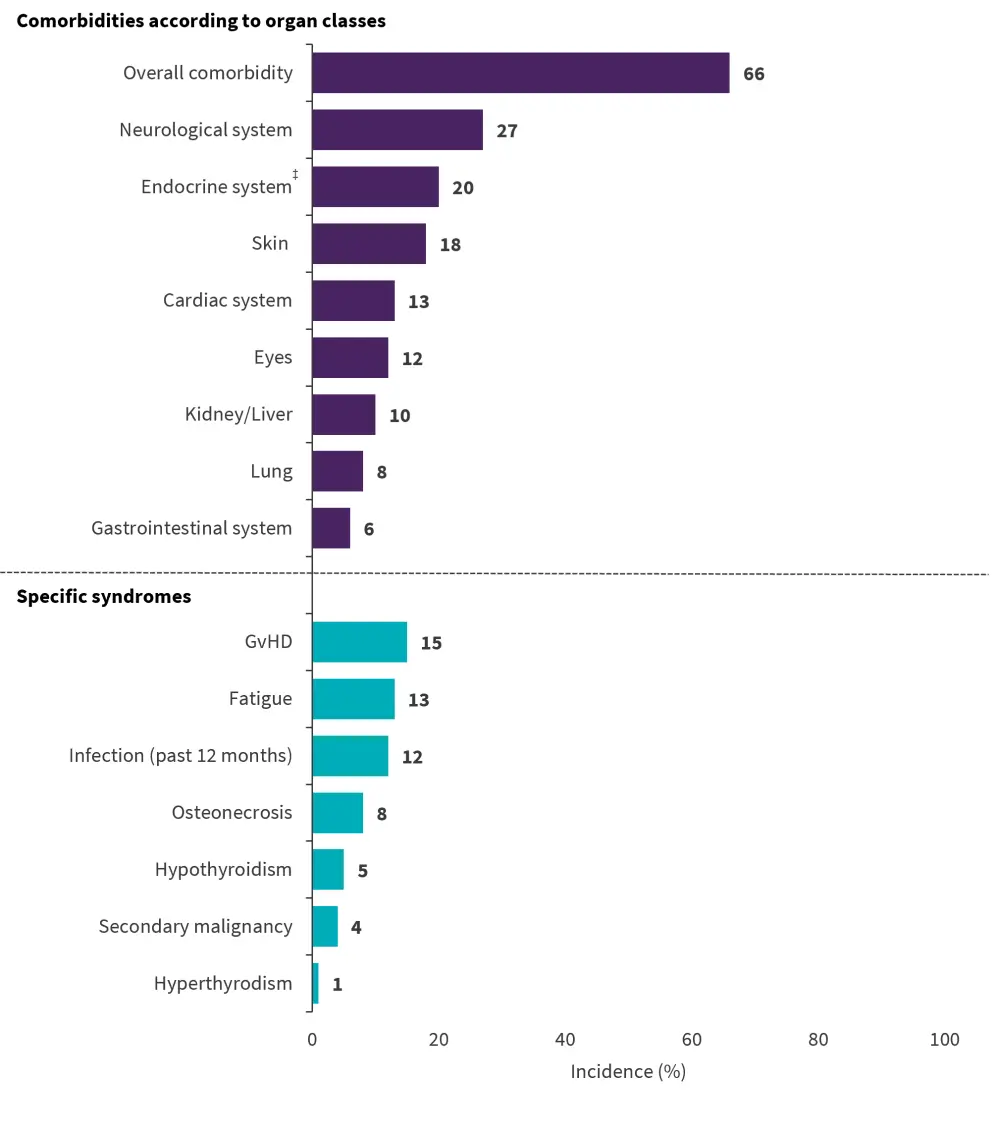
GvHD, graft versus host disease
*Data from Gokbuget, et al.1
†Evaluable patients per condition were as follows: N = 538 for overall comorbidities, skin, lung, cardiac system, neurological system, kidney/liver, GvHD, osteonecrosis, secondary malignancy and hyperthyroidism; N = 537 for gastrointestinal system, eyes, and hypothyroidism; N = 533 for infections and fatigue; N = 211 for female endocrine disorders; and N = 327 for male endocrine disorders.
‡Average for male and female patients.
Characteristics of specific syndromes
Among patients with GvHD, involvement of one, two, and two or more organs occurred in 50%, 30%, and 19%, respectively; the manifestations of which included skin involvement (n = 52), eye impairments (n = 45), liver affection (n = 25), intestine disorder (n = 12), or lung dysfunction (n = 10). The median time from diagnosis to GvHD event was 3 years. Osteonecrosis occurrences were Grade 1−2, 3, or 4 in 18%, 48%, 35%, and 2%, respectively, and involved the hip (n = 18), shoulder (n = 8), or both joints (n = 2). The median time from diagnosis to GvHD documentation and osteonecrosis event was 3 years.
For secondary malignancies, the median time from diagnosis to event was 11 years. The type of tumors observed included melanoma (n =4), basal cell carcinoma (n = 4), hematological malignancies (n = 4), breast cancer (n = 2), prostate cancer (n = 2), and one case each of glioblastoma, small intestine cancer, cancers of stomach, cervix, and sarcoma.
The incidence of fatigue was mostly Grade 1−2 (n = 63); Grade 3 in seven patients and Grade 4 in one, with a median age and time to event of 35 years and 3.5 years, respectively. Infections were mostly respiratory involvement.
Characteristics of comorbidities according to organ classes
Of the 27% of patients with neurological disorders, 13% had mood alterations, 7% had cognitive disturbances, and 7% had peripheral sensory and/or motor neuropathy. Among the 20% with an endocrine disorder (24% in female and 17% in male), infertility occurred in 17% of females aged <40 years, 4% had osteoporosis, and 5% had diabetes. In males, 4% had infertility, with an additional eight experiencing pathological hormone status and/or erectile dysfunction, and diabetes in 2%.
The most commonly reported skin disorder was alopecia in 10% of patients. The most frequent eye comorbidities were cataract in 6%, conjunctivitis in 4%, and visual impairment in 3% of patients.
Factors associated with general health status and comorbidities
Overall, SCT was significantly associated with ECOG PS status and comorbidity incidence. Patients who underwent SCT vs those not receiving SCT had an ECOG PS of 0−1 in 86% vs 98%, respectively (p < 0.0001); and a higher incidence of comorbidities (p<0.0001). Moreover, univariate analyses revealed the significant impact of SCT across all comorbidities except cardiac disease, and for a few specific syndromes: GvHD, infections, and fatigue. This was confirmed by multivariate analysis.
A higher proportion of younger vs older (>55 years) patients achieved an ECOG PS status of 0−1 (p = 0.02), with older patients experiencing a higher incidence of overall comorbidities (p = 0.05), cardiac disorders (p = 0.03), endocrine disorders in male patients (p = 0.04), and secondary malignancies (p = 0.04); however, this was not significant.
Whilst gender did not impact ECOG PS, females vs males had significantly higher incidences of any comorbidity (p = 0.03), neurological disorders (p = 0.01), skin comorbidities (p = 0.02), and infections (p = 0.008). The trial cohort was not associated with ECOG PS, but significantly correlated with overall comorbidities, GvHD occurrence (p < 0.0001) and secondary malignancies (p = 0.003) in multivariate analyses (Table 2).
Table 2. Factors associated with health status and comorbidity incidences*
|
CT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group Performance Status; F, female; GI, gastrointestinal; GvHD, graft versus host disease; m, male; SCT, stem cell transplantation |
|||||||||
|
Health condition and comorbidities, % |
SCT vs CT |
Gender |
Age |
Trials |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
|
SCT |
CT |
Male |
Female |
≤55 years |
>55 years |
2–4 |
5−7 |
|
|
ECOG PS 0–1 |
86 |
98 |
94 |
95 |
95 |
84 |
96 |
94 |
|
|
>1 comorbidity |
87 |
57 |
62 |
72 |
65 |
77 |
71 |
65 |
|
|
Skin |
32 |
12 |
15 |
23 |
18 |
19 |
15 |
19 |
|
|
Lung |
18 |
3 |
9 |
6 |
8 |
8 |
4 |
9 |
|
|
Cardiac system |
16 |
12 |
13 |
14 |
12 |
27 |
21 |
11 |
|
|
GI system |
9 |
4 |
7 |
4 |
5 |
12 |
4 |
6 |
|
|
Neurological system |
36 |
23 |
24 |
33 |
27 |
42 |
31 |
26 |
|
|
Kidney/liver |
23 |
5 |
10 |
11 |
10 |
19 |
7 |
11 |
|
|
Endocrine system (f) |
38 |
17 |
— |
24 |
23 |
33 |
15 |
26 |
|
|
Endocrine system (m) |
34 |
9 |
17 |
— |
16 |
35 |
12 |
18 |
|
|
Eye impairment |
29 |
5 |
11 |
14 |
12 |
23 |
9 |
13 |
|
|
Infection |
20 |
8 |
9 |
17 |
12 |
12 |
11 |
12 |
|
|
Fatigue |
19 |
11 |
12 |
15 |
13 |
15 |
9 |
14 |
|
|
GvHD |
47 |
0 |
14 |
16 |
15 |
15 |
2 |
18 |
|
|
Osteonecrosis |
9 |
7 |
6 |
10 |
8 |
0 |
10 |
7 |
|
|
Secondary malignancies |
4 |
4 |
4 |
3 |
4 |
12 |
9 |
3 |
|
|
Hypothyroidism |
6 |
4 |
3 |
7 |
5 |
0 |
4 |
5 |
|
|
Hyperthyroidism |
1 |
1 |
2 |
1 |
1 |
0 |
2 |
1 |
|
Conclusion
This study highlights the spectrum of comorbidities, late effects of disease, and treatment in long-term survivors of adult ALL, as well as the higher risk of comorbidities in patients receiving transplantation. These retrospective data are beneficial to inform aftercare procedures and can also provide a reference for patient-centered endpoints in future trials. Future prospective studies on the long-term observations of patients in clinical trials are warranted to fully elucidate the spectrum of treatment-related effects, improve patient care, and evaluation of new treatment approaches.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

