All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Long-term data with CD19 CAR T-cell therapy in children and young adults with B-ALL
CD-19-directed chimeric antigen receptor (CAR) T-cell therapy has demonstrated highly encouraging results in children and young adults (CAYAs) with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). However, relapse rates are still high and long-term follow-up data are limited. In the R/R setting, allogeneic hematopoietic stem cell transplantation (allo-HSCT) along with chemotherapy, is associated with improved disease-free survival in the long-term; however, the value of consolidative allo-HSCT following CD19 CAR T-cell therapy in CAYAs with R/R B-ALL is unknown.1
Nirali N. Shah and colleagues conducted a phase I study of CD19.28ζ CAR T-cells in this patient population to investigate whether alternative chemotherapy regimens could reduce disease burden more effectively. Intensified lymphodepletion strategies were also used. Results with a long-term follow-up of 4.8 years from 50 patients were published in the Journal of Clinical Oncology.1
This was a single center, phase I study (NCT01593696) of CD19.28ζ CAR T-cells (an anti-CD19 single-chain variable fragment plus TCR zeta and CD28 signaling domain).2 In the dose-escalation phase, the primary objective was to define the maximum tolerated dose, discover the toxicity profile, and the feasibility of CD19 CAR T-cells. Twenty-one patients were treated in this phase and the results were previously published in The Lancet.2 In brief, CD19.28ζ CAR T-cells demonstrated a 90% feasibility, the maximum tolerated dose was 1 × 106 cells/kg, and all toxicities were reversible.2
Methods
- In this current analysis, 30 additional patients were treated in an expansion phase and results from a total of 50 patients were reported. A retrospective analysis was performed to investigate the impact of allo-HSCT on survival.
- Study design was as follows:
- Dose levels (DL): 1 × 106 cells/kg once on Day 0 (DL1) and 3 × 106 cells/kg once on Day 0 (DL2)
-
- To reduce disease burden prior to CAR-T infusion, lymphodepletion regimens were evaluated in patients, as seen in Figure 1 below.
Figure 1. Lymphodepletion regimens used in the study*
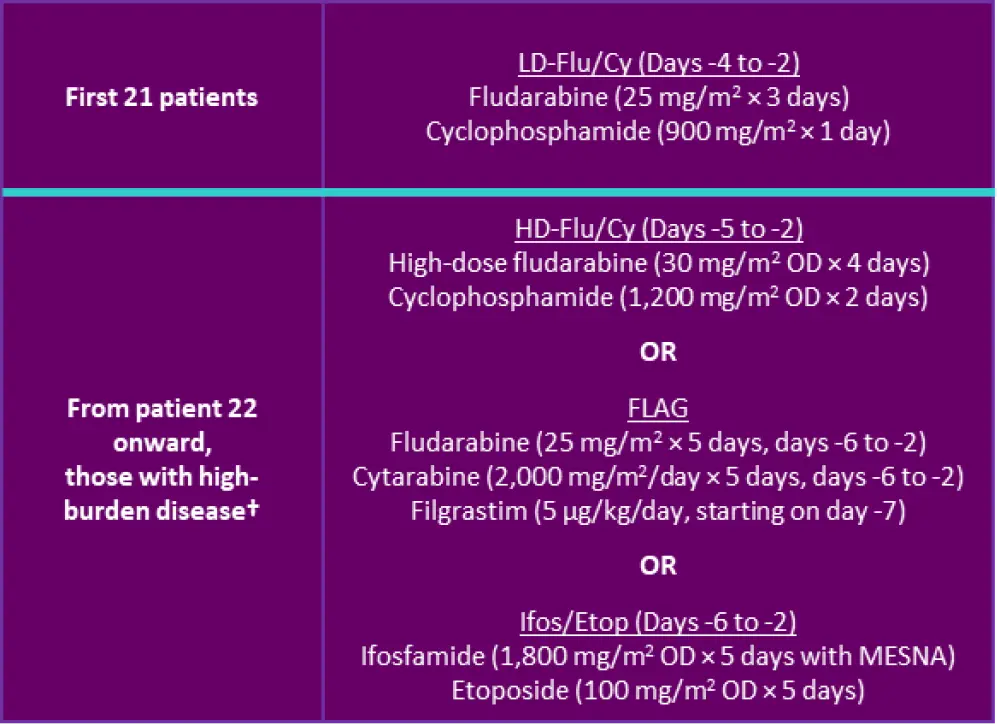
Cy; cyclophosphamide; Flu, fludarabine; HD, high dose; LD, low dose; MESNA, 2-mercaptoethane sulfonate; OD, once daily.
*Adapted from Shah et al.1
†High burden disease was defined by ≥25% bone marrow blasts, circulating peripheral blasts or lymphomatous disease.
Patients
A total of 53 patients were enrolled in the study. All but two patients (who had diffuse large B-cell lymphoma, and who were excluded from these analyses) had B-ALL. One patient with B-ALL could not receive CAR T-cell infusion due to invasive fungal disease. Baseline characteristics of patients with B-ALL who were included in the analyses (n = 50) are presented in Table 1. Forty-five patients were treated at DL1 and five patients were treated at DL2.
Table 1. Baseline characteristics*
|
CAR, chimeric antigen receptor; CNS, central nervous system; CSF, cerebrospinal fluid; HSCT, hematopoietic stem cell transplantation; WBC, white blood cells. †More than 5 WBC/µL in CSF and cytospin-positive for blasts. |
|
|
Characteristic |
N = 50 |
|---|---|
|
Median age, years (range) |
13.5 (4.3−30.4) |
|
Male, n (%) |
40 (80) |
|
Median prior regimens, n (range) |
4 (1−16) |
|
Primary refractory, n (%) |
11 (22) |
|
Prior HSCT, n (%) |
22 (44) |
|
Prior CD19-targeted therapy, n (%) |
7 (14) |
|
≥5% marrow blasts, n (%) |
32 (64) |
|
Extramedullary disease, non-CNS, n (%) |
4 (8) |
|
CNS disease, n (%) |
13 (26) |
Results
Safety
Any grade of cytokine release syndrome (CRS) was reported in 35 patients (70%), and nine patients (18%) experienced Grade 3−4 CRS (Table 2). The median time to CRS onset was 5 days (range, 1−12 days). All events were resolved.
Table 2. Safety outcomes*
|
CRS, cytokine release syndrome †Includes seizure and Grade 3 dysphasia. |
|
|
Outcome, % |
Patients (N = 50) |
|---|---|
|
Grade 3 CRS |
12 |
|
Grade 4 CRS |
6 |
|
CRS management |
|
|
Neurotoxicity |
20 |
There was an association between disease burden and CRS severity. Among responders who received low-dose fludarabine/cyclophosphamide (Flu/Cy), Grade 3−4 CRS events were significantly higher in patients with an ≥M2 marrow (≥5% marrow blasts) compared with those who had an M1 marrow (<5% marrow blasts) (57.1% vs 6.67%, p = 0.005). Eight of nine patients who had Grade 3−4 CRS had ≥M2 marrow blasts. Grade 3−4 CRS and ≥M2 marrow blasts were reported in 70% and 80% of patients with neurotoxicity, respectively.
Efficacy
Of 50 patients, 31 (62.0%) achieved complete response (CR). Of those with CR, 28 patients (90.3) had minimal residual disease (MRD) negativity, corresponding to an overall MRD negativity of 56.0%.
CR rates were higher in patients who:
- were primary refractory (p = 0.0035)
- had fewer prior lines of therapy (p = 0.033)
- had an M1 marrow (MRD-positive, <5% marrow blasts; p = 0.0007)
- received any Flu/Cy regimen for lymphodepletion (p = 0.041)
- CR rates with low-dose and high-dose Flu/Cy were 71.4% and 57.1%, respectively
- had low-burden disease (M1 marrow) (94.1% vs 45.5% with high-burden disease defined as ≥M2 marrow; p = 0.0007)
All patients with central nervous system (CNS) involvement who had CRS could be treated effectively, and patients achieved marrow response.
Higher CAR T-cell expansion and Grade 3−4 CRS were associated with CR; response rates did not differ with expression of the T-cell exhaustion markers on infused CAR T-cells. CD4+ and CD8+ CAR T-cell populations were balanced between central and effector immunophenotypes. Cytokine peak levels were differentially increased among those with low- and high-grade CRS.
One patient with prior exposure to a CD19 CAR T-cell therapy and four patients with prior exposure to blinatumomab failed to respond to the study treatment.
Long-term outcomes
Table 3 summarizes survival with a median follow-up of 4.8 years (range, 3.5−7.2 years). In the high-burden disease cohort, eight of 15 patients who achieved CR proceeded to allo-HSCT.
Table 3. Survival outcomes*
|
CI, confidence interval; EFS, event-free survival; M1 marrow, <5% marrow blasts; M2 marrow, ≥5% marrow blasts; NR, not reached; OS, overall survival. *Adapted from Shah et al.1 |
|
|
Outcome |
Patients (N = 50) |
|---|---|
|
Median OS, months (95% CI) |
10.5 (6.3−29.2) |
|
Median EFS, months (95% CI) |
3.1 (0.9−7.7) |
|
3-month EFS, % (95% CI) |
52 (37.4−64.7) |
|
6-month EFS, % (95% CI) |
38 (24.8−51.1) |
The impact of consolidative HSCT
Of 28 patients who achieved MRD-negative CR, 21 (75%) underwent allo-HSCT for consolidation; median time to transplantation from CAR infusion was 54 days (range, 42−97 days).
- Median overall survival (OS) from allo-HSCT Day 0 was 70.2 months (95% CI; 10.4 to not-estimable)
- Median event-free survival (EFS) was not reached
- 5-year EFS after allo-HSCT was 61.9% (95% CI, 38.1−78.8)
Eight patients died between 0.8 and 71 months after allo-HSCT due to transplant-associated complications, graft-versus-host disease (GvHD), infection, secondary malignancy 3 years following transplant, and relapse. Cumulative incidence of relapse posttransplant was 4.8% and 9.5% at 12 and 24 months, respectively.
Patients who did not underwent transplantation (n = 7) relapsed at a median of 152 days (range, 94−394) after CAR infusion, emphasizing the role of consolidative allo-HSCT.
Conclusion
This study represents the longest follow-up after CD19 CAR T-cell therapy for B-ALL. A consolidative allo-HSCT after CAR infusion was associated with:
- a relapse rate of <10% at 24 months
- 5-year EFS of 61.9%, and
- a median OS of 70.2 months
In managing B-ALL with CNS involvement, the findings indicate that CNS2 and CNS3 disease can be treated safely and effectively, and support further evaluation of CAR T-cells in this setting. Lymphodepletion with a Flu/Cy-based regimen was associated with improved responses over alternative regimens. Results also indicate that prior CD19-targeted therapies may negatively impact response to CD19 CAR T-cell therapy. Overall, CD19.28ζ CAR T-cell therapy followed by transplant may have a potential for long-term durable disease control in CAYAs with R/R B-ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

