All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Long-term outcomes of inotuzumab ozogamicin plus allo-HSCT conditioning regimen in CD22+ lymphoid malignancies
Do you know... In the comparison study of inotuzumab ozogamicin plus bendamustine/fludarabine/rituximab (BFR) conditioning regimen vs BFR alone for CD22+ lymphoid malignancies, what was the 5-year progression-free survival rate in the study vs control cohort?
Inotuzumab ozogamicin (InO) is an anti-CD22 monoclonal antibody that has demonstrated anti-tumor activity and manageable safety in patients with B-cell acute lymphoblastic leukemia (ALL) and lymphoma; however, it is associated with veno-occlusive disease, particularly in patients undergoing allogeneic stem cell transplantation (allo-SCT) receiving two-alkylating agent conditioning regimen or those with an elevated bilirubin level at or above the upper limit of normal pre-SCT.1
Here, we summarize a presentation by Khouri from the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, on the long-term survival results of InO plus an allo-HSCT conditioning regimen in patients with relapsed/refractory CD22+ lymphoid malignancies.
Study methods
This study included patients aged 18–70 years with relapsed/refractory chronic lymphocytic leukemia or non-Hodgkin lymphoma (NHL) who had an Eastern Cooperative Oncology Group Performance Status of ≤2, serum bilirubin <2 mg/dL, and alanine transaminase (AST) <2 times the upper limit of normal.
InO was given on Day 13 prior to the one-alkylator containing conditioning regimen of bendamustine/fludarabine/rituximab (BFR). Patients received tacrolimus and methotrexate for graft-versus-host disease (GvHD) prophylaxis.
Results
In total, 26 patients were included in the study; baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
Characteristic, % (unless otherwise stated) |
InO + BFR |
|
Median age (range), years |
59 (26–70) |
|
≥60 years |
46 |
|
HCT-CI ≥3 |
42 |
|
Histology |
|
|
CLL |
42 |
|
MCL |
31 |
|
FL |
19 |
|
DLBCL |
8 |
|
Median prior lines of therapies (range), n |
2.5 (1−6) |
|
Prior autologous transplant |
4 |
|
Prior therapies |
|
|
Ibrutinib |
38 |
|
Venetoclax |
19 |
|
Idelalisib |
8 |
|
Nivolumab |
4 |
|
CAR T cell |
4 |
|
Disease status at transplant |
|
|
CR |
69 |
|
PR |
27 |
|
SD |
4 |
|
InO dose level |
|
|
0.6 mg/m2 |
15 |
|
1.2 mg/m2 |
8 |
|
1.86 mg/m2 |
77 |
|
Donor type |
|
|
MUD |
58 |
|
MSD |
42 |
|
BFR, bendamustine, fludarabine, rituximab; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CR, complete remission; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HCT-CI, hematopoietic cell transplantation – comorbidity index; InO, inotuzumab ozogamicin; MCL, mantle cell lymphoma; MUD, matched unrelated donor; MSD, matched sibling donor; PR, partial response; SD, stable disease. |
|
Graft-versus-host disease, treatment-related mortality, and response rates
Overall, 42% of patients did not have an absolute neutrophil count of <0.5 × 109/L, and 77% did not have a platelet count of <20 × 109/L. All patients had donor cell engraftment, with a median absolute neutrophil count recovery of 6.5 days and a median platelet recovery of 0 days.
Grade 2–4 acute GvHD occurred in 27% of patients, with Grade 3–4 in only 4%. The incidence of chronic GvHD was 50%. The 1- and 5-year treatment-related mortality was 12%.
Of the seven patients with chronic lymphocytic leukemia who had a partial response and stable disease at study entry, six achieved a CR post-allo-HSCT, with four patients achieving MRD-negativity. Among the four patients with CR at the time of transplant, three attained MRD negativity.
Survival and toxicities
At the median follow-up of 48.7 months, the 5-year overall survival (OS) and progression-free survival (PFS) were 84% and 80%, respectively. For patients receiving matched sibling donors (MSD) vs those with matched unrelated donors, the OS was 100% vs 72%, respectively; and the PFS was 100% vs 64%, respectively. There was a total of four deaths in study, due to acute GvHD (n = 2), cGvHD (n = 1), and progressive disease (n = 1).
Comparison analysis
For the comparison analysis between the BFR + InO study group and the historical control group (N = 56), there were no differences in the patient characteristics. Overall, there was a trend towards a better OS and PFS for patients with CD22+ NHL in the study vs the control group (Figure 1).
There was one case of veno-occlusive disease reported in the study group. Grade 1 AST increase was seen in nine patients within the control group and one patient in the study group; and Grade 2 AST increase was seen in one patient in each of the control and study group. Grade 1 and 2 bilirubin increase occurred in five and two patients, respectively, within the control group; and one patient each experienced Grade 1, 2, and 3 bilirubin increase in the study group.
Figure 1. 5-year OS and PFS in the study vs control group*
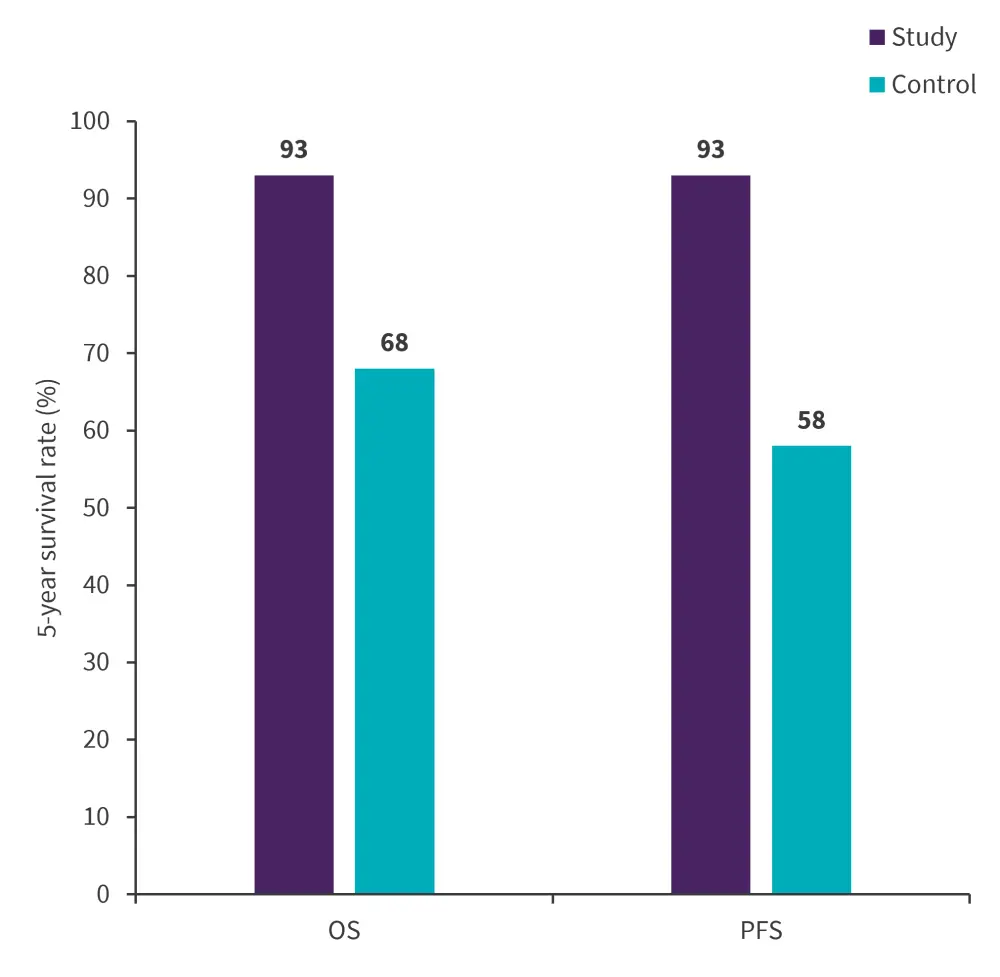
OS, overall survival; PFS, progression-free survival.
*Adapted from Khouri.1
Presenter’s conclusion
This study showed that InO combined with BFR is a safe regimen that may improve survival outcomes for patients with CD22+ NHL; however, these data need to be validated in a larger cohort. Additional studies are ongoing investigating the efficacy and safety of InO with posttransplant cyclophosphamide to reduce the risk of GvHD in patients with CD22+ diseases, such as those with ALL undergoing HSCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

