All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Old and new ADCs for the treatment of ALL and AML
Since the beginning of their development in the early 1980s, a lot of progress has been made in the development of monoclonal antibodies (mAbs) for cancer treatment thanks to the innovations in cancer biology and bioengineering.
During the 3rd Annual Meeting of the International Academy for Clinical Hematology (IACH), Norbert Vey gave an overview of the current mAbs under development for the treatment of acute lymphocytic leukemia (ALL) and acute myeloid leukemia (AML). Here, we report a summary of his talk.1
Types of mAb for cancer treatment can be distinguished in:
- Unconjugated (often with limited activity in acute leukemias)
- Conjugated with chemotoxin
- Conjugated with immunotoxin
- Bispecific (i.e., CD19 and CD23 T-cell engagers)
Antibody–drug conjugates1
In his talk, Norbert Vey focused on antibody–drug conjugates (ADCs), constructs in which a cytotoxic drug is attached by linkers to a monoclonal antibody that targets a specific antigen exclusively found on cancer cells (Figure 1). When the ADC binds to the target cell, the complex is internalized and the cytotoxic drug released, inducing DNA damage and subsequent cell death.
Among the approved ADCs, only two have been approved for the treatment of acute leukemia. These are gemtuzumab ozogamicin (GO) for newly diagnosed CD33+ AML and inotuzumab ozogamicin for relapsed or refractory (R/R) CD22+ B-cell precursor ALL.
The three most critical components in the development of novel active ADCs are the target, the linker, and the conjugated payload. An optimal target should be specific, accessible, widely expressed, internalized, and expressed by leukemic stem cells (Figure 1). Among multiple suggested ADC targets for AML, the most widely used are CD33 and CD123. Although they are both expressed by leukemic cells, they are also expressed by normal hematopoietic stem cells, explaining the observed myelosuppression seen with anti-CD33 ADCs (i.e., GO) and highlighting the narrow therapeutic index of such ADCs. Other targets like IL-1RAP, expressed by leukemic cells but not by normal hematopoietic cells, are attractive candidates for novel ADCs.
With regards to the optimal ADC linker, this must be stable in the circulation, should be hydrolyzable within the cell, and should lead to a high ADC/unconjugated mAb ratio. The ideal payload must have a high cytotoxic activity, must be hydrophilic, and able to evade efflux pumps (Figure 1). New payloads are now available that meet the above criteria and are currently under investigation in AML. Such payloads are the pyrrolobenzodiazepine dimer (PBD), DGN-549, and monomethyl auristatin E (MMAE).
Figure 1. Schematic representation of an ADC and the parameters needed for optimal activity/safety (Adapted from Vey N, IACH 20201)
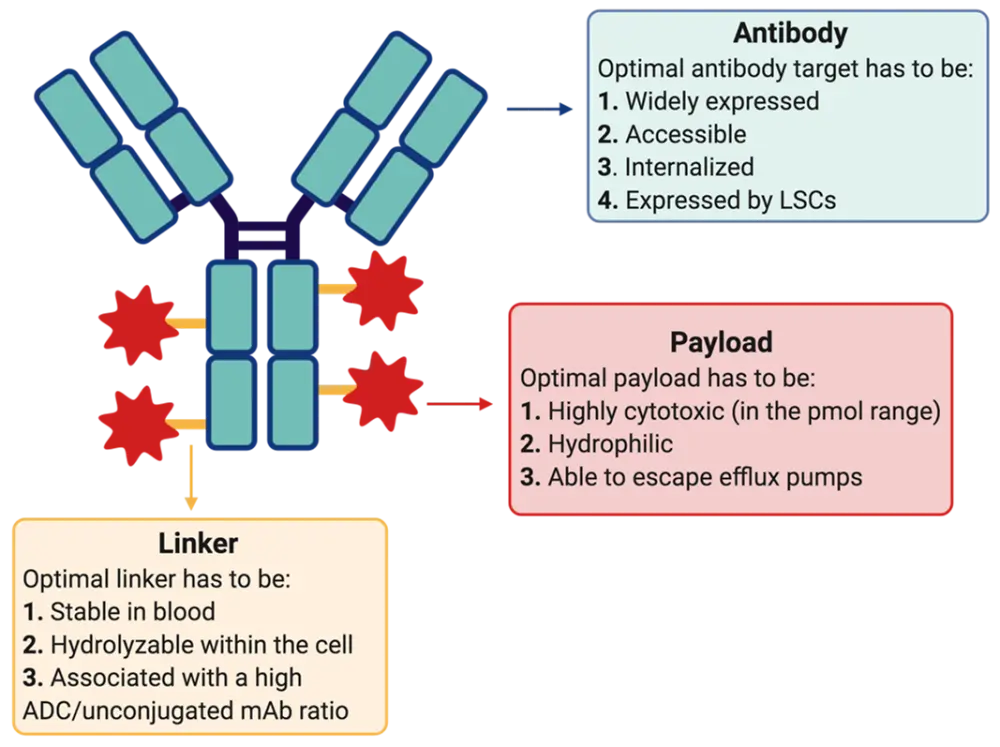
ADC, antibody–drug conjugate; LSC, leukemic stem cell; mAb, monoclonal antibody.
ALL – Inotuzumab ozogamicin1
Inotuzumab ozogamicin (IO) is an anti-CD22 mAb conjugated to calicheamicin, an intercalating agent causing DNA damage and cell death. The approval of this ADC was based on data from the INO-VATE phase III trial (NCT01564784) that compared the efficacy of IO versus investigator's choice of chemotherapy in 326 patients with R/R CD22+ B-cell precursor ALL. The primary endpoints of complete remission (CR) and overall survival (OS) were met, with patients in the IO group versus standard-therapy group having2:
- Significantly higher CR rate: 80.7% (95% CI, 72.1─87.7) versus 29.4% (95% CI, 21.0─38.8; p < 0.001)
- Better median OS: 7.7 months (95% CI, 6.0─9.2) versus 6.7 months (95% CI, 4.9─8.3); HR = 0.77 (97.5% CI, 0.58─1.03; p = 0.04)
- Longer median duration of remission: 4.6 months (95% CI, 3.9─5.4) versus 3.1 months (95% CI, 1.4─4.9); HR = 0.55 (95% CI, 0.31─0.96; p = 0.03)
- Longer median progression-free survival (PFS): 5.0 months (95% CI, 3.7─5.6) versus 1.8 months (95% CI, 1.5─2.2); HR = 0.45 (97.5% CI, 0.34─0.61; p < 0.001)
ALL – Other ADCs in development
Ongoing studies are evaluating IO in combination with chemotherapy in the frontline treatment of ALL. Other ADCs investigated for the treatment of ALL are shown below in Table 1.
Table 1. ADCs under investigation for ALL1
|
ADC, antibody-drug conjugate; ALL, acute lymphocytic leukemia; IL7Ra, interleukin 7 receptor a; MMAE, monomethyl auristatin E; ORR, overall response rare; PBD, pyrrolobenzodiazepine dimer; R/R, relapsed/refractory. |
||||
|
ADC |
Target |
Cytotoxic payload |
Clinical trial |
Comment |
|---|---|---|---|---|
|
Denintuzumab mafodotin (SGN-CD19A) |
CD19 |
DNA-interacting PBD |
Phase I, R/R ALL, 35% ORR |
|
|
Loncastuximab tesirin |
CD19 |
DNA-interacting PBD |
Phase I, early termination due to limited efficacy |
|
|
VLS-101 |
ROR1 |
Tubulin-binding MMAE |
Phase I, ongoing |
|
|
INA-03 |
CD71 |
Tubulin-binding MMAE |
Phase I, ongoing |
|
|
B12-MMAE |
IL7Ra |
Tubulin-binding MMAE |
N/A |
Preclinical stage |
AML – Gemtuzumab ozogamicin1
The first approved ADC for the treatment of AML was gemtuzumab ozogamicin (GO), which targets CD33 and is also conjugated to calicheamicin. This was first licensed in 2000 but was withdrawn from the market in 2010 due to safety concerns. Meta-analysis of all the major clinical trials and long-term follow-up studies led to its re-approval in 2017 by the U.S. Food and Drug Administration (FDA) in combination with standard chemotherapy due to its significant survival benefit for adult patients with newly diagnosed or R/R CD33+ AML, which was extended to pediatric patients in 2020 (read more here).
To date, specific safety recommendations for the management of GO in AML have been published and help guide treatment. These have been summarized here. Moreover, from this extensive 10-year clinical experience with GO, several important parameters have been identified with regards to the safe use and management of ADCs. Firstly, ADC pharmacokinetics are critical and should be identified as early as possible. In the case of GO, increased clearance was seen with subsequent infusions and a 90% target saturation upon first dose, which was associated with rapid CD33 cell surface recycling. Such data provided the rationale for the development and use of fractionated GO doses. Predictive factors of response are also important and from the GO experience we know that benefit is restricted to patients with favorable- or intermediate-risk and that higher CD33 expression is linked to greater benefit. The main limitations of GO treatment include the associated myelosuppression and liver toxicity.
AML – Novel ADC development1
Several novel ADCs have been developed for the treatment of AML. The most important ones are shown below in Table 2.
Table 2. Novel ADCs under clinical development for AML1
|
ADC, antibody-drug conjugate; DM4, N20-deacetyl-N20-(4-mercapto-4-methyl-1-oxopentyl)maytansine; IGN, indolinobenzodiazepine pseudodimer; IQB, isoquinolidinobenzodiazepine; PBD, pyrrolobenzodiazepine dimer. |
||||
|
ADC name |
Target |
Payload |
Linker |
Development stage |
|---|---|---|---|---|
|
Vadastuximab talirine (SGN-CD33A) |
CD33 |
PBD |
Dipeptide linker (protease-cleavable) |
Phase III |
|
IMGN-779 |
CD33 |
DGN-462 |
Disulfide linker |
Phase I |
|
AVE9633 (huMy9-6-DM4) |
CD33 |
DM4 |
Disulfide linker |
Phase I (terminated) |
|
IMGN-632 |
CD123 |
IGN |
Dipeptide linker (protease-cleavable) |
Phase I |
|
SGN-CD123A |
CD123 |
PBD |
Dipeptide linker (protease-cleavable) |
Phase I (terminated) |
|
CLT030 |
CLL-1 |
IQB |
Dipeptide linker (protease-cleavable) |
Preclinical |
|
Anti-CLL-1 ADC |
CLL-1 |
PBD dimer |
Disulfide linker |
Preclinical |
AML – Vadastuximab talirine (SGN-CD33A)
Most of the novel ADCs under investigation for AML target CD33 or CD123 despite the therapeutic limitation of such targets as mentioned above. Of these ADCs, the one with the most clinical experience to date is vadastuximab talirine (SGN-CD33A), which targets CD33, has a cleavable dipeptide linker that is highly stable in circulation, and carries a PBD payload that crosslinks DNA inducing cell death (Table 2).
Data from two phase I studies reported encouraging efficacy for vadastuximab talirine when used as frontline either alone or in combination with hypomethylating agents in patients with treatment-naïve CD33+ AML. These trials were both presented during the 2016 American Society for Hematology (ASH) Annual Meeting and Exposition. The first trial by Bixby Dale and colleagues3 administered vadastuximab talirine in 26 older patients and reported 30- and 60-day mortality rates of 0% and 15%, respectively. The combined CR and CR with incomplete hematological recovery (CRi) rate was 54%, with 19% of patients achieving morphologic leukemia-free status. Most adverse events were manageable with the main safety limitation being myelosuppression.
In the other phase I trial (NCT01902329) by Fathi Amir and colleagues,4 vadastuximab talirine was combined with hypomethylating agents for the treatment of 53 patients who had declined intensive therapy. The reported CR/CRi rate was 73% and the 30- and 60-day mortality rates, 2% and 8%, respectively. Importantly, 47% of responders achieved measurable disease negativity as assessed by flow cytometry. For more information on the study design and outcomes of this trial, please read here. Leading on from these results the phase III CASCADE trial (NCT02785900) was initiated but quickly terminated due to excessive toxicity, including higher mortality rates and fatal infections.
AML – IMGN-6321
IMGN-632 is a high affinity CD123-targeting ADC that has a stable cleavable linker and is conjugated to the novel payload DGN549, which cleaves DNA and produces single strand breaks (Table 2). Preliminary data from the first-in-human phase I trial (NCT03386513) of IMGN-632 were presented at ASH 2018 by Daver Naval (read more here).5 This novel ADC was administered intravenously (escalating dose: 0.015–0.45 mg/kg) in 95 patients with CD123+ R/R AML or blastic plasmacytoid dendritic cell neoplasm that had received > 3 prior therapies. IMGN-632 demonstrated a tolerable safety profile with the most common treatment-related adverse events being infusion-related reactions, neither of which required treatment discontinuation (24% all grades; 8% Grade 3). For patients with R/R AML (n = 71), the CR/CRi rate was 18% with 54% of patients having a reduction in bone marrow blasts. The 30-day mortality rate was 6%. Although the response rates might seem low, it should be noted that 92% of the patients included in this study had failed prior intensive therapies and were heavily pre-treated and thus IMGN-632 is a promising treatment for this patient subset.6 In addition, IMGN-632 seems to show promising efficacy in high-risk patients (see interview for more information).
Conclusion
Wide clinical experience with IO and GO has shown that ADCs can be promising therapies for patients with ALL or AML when managed accordingly for adverse event minimization. With the current advances in ADC biology and engineering, the optimal parameters for ADC components (target, linker, payload) have been identified and are used in the development of novel agents. Multiple novel ADCs, like IMGN-632, are currently under clinical or preclinical investigation (Table 2) with the hope of providing increased efficacy and reduced toxicity in the treatment of AML and ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

