All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Prevention and management of relapse after CAR T-cell therapy in B-ALL
Question 1 / 2
Which of the following prevention strategies for post chimeric antigen receptor T-cell therapy relapse can be used to minimize the risk of antigen escape?
A
Humanized CD19-targeted CAR T-cell constructs
B
Consolidative hematopoietic stem cell transplantation
C
Dual-targeted CAR T-cell therapies
D
T-cell antigen-presenting cells
Despite the excellent outcomes following CD19-directed chimeric antigen receptor (CAR) T-cell therapy in relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL), approximately 50% of children and young adults experience relapse within the first year after infusion; this is associated with poor prognosis given the limited immunotherapeutic options for this chemotherapy-refractory population.1
There is an urgent need to identify risk factors associated with an increased risk of relapse to standardize relapse prevention strategies in high-risk patients, as well as salvage options following a relapse. Below, we highlight the emerging strategies and future directions in the prevention and management of post CAR T-cell therapy relapse in children and young adults with B-ALL.1
Post CAR T-cell therapy monitoring: B-cell aplasia vs next-generation sequencing1
In general, functional CAR T-cell persistence is a requirement for long-term durable remissions. Assays that directly detect the number of CAR T cells have been shown to predict long-term outcomes; however, this approach is limited by the low sensitivity, high variability in detection across trials, and limited availability.
Although B-cell aplasia is a promising biomarker of functional CAR T-cell persistence, it has several limitations including its variable definitions across trials and institutions, differences in timing of B-cell recovery in peripheral blood vs bone marrow, no consensus standard for the frequency of monitoring, and its inability to predict CD19-negative relapse or lineage switching.
Next-generation sequencing for measurable residual disease (MRD) is a highly sensitive assay that can be used to identify patients at high-risk for relapse. Patients with positive MRD at multiple or a single timepoint between 3 and 6 months following CD19 CAR T-cell therapy always experience relapse. Compared with MRD by flow cytometry, which has a median time to relapse of 1–2 months, next-generation sequencing-MRD facilitates a median time of 6 months, allowing for prevention interventions. Limitations of this assay include the lack of surface antigen characterization.
Relapse prevention1
Strategies currently being explored to prevent relapse include modifying preinfusion risk factors, optimizing CAR design to enhance CAR T-cell persistence, use of next-generation CAR T cell strategies to minimize CD19 escape, and the use of consolidative hematopoietic stem cell transplantation (HSCT) and non-HSCT approaches to prolong durable remissions (Figure 1).
Figure 1. Post CAR T-cell therapy relapse prevention strategies*
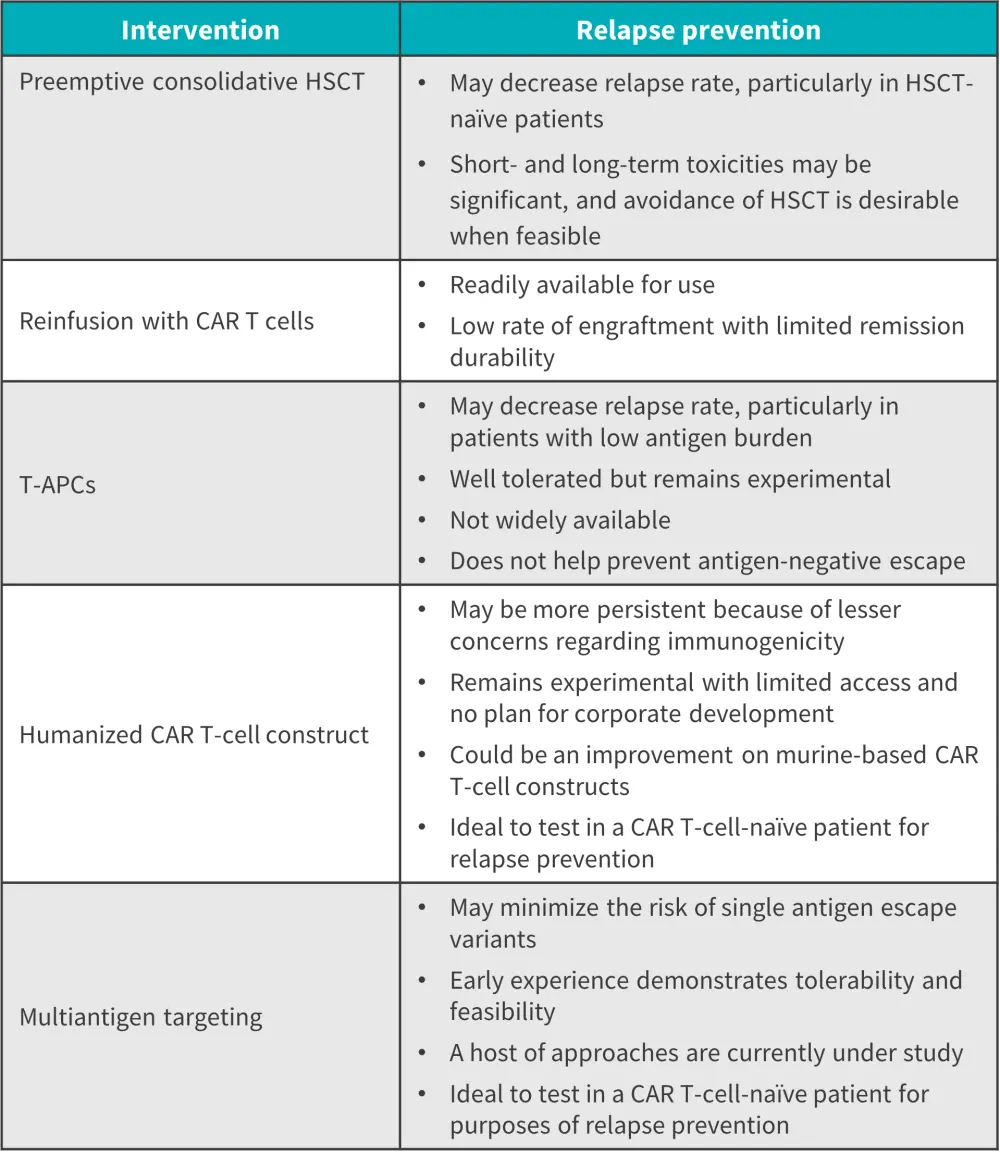
APC, antigen-presenting cell; CAR, chimeric antigen receptor; HSCT, hematopoietic stem cell transplantation; LS, lineage switch; T-APC, T-cell antigen-presenting cell.
*Data from Lamble, et al.1
Modifying preinfusion risk factors1
Product, disease, and patient factors affect CAR T-cell outcomes, and modifying them could reduce relapse risk. Prior treatment to T-cell apheresis should be minimized to optimize CAR T-cell fitness. A recent CAR T-Cell Multicenter Analysis consortium found that prior exposure to blinatumomab did not impact CD19 CAR T-cell outcomes, but prior blinatumomab failure correlated with a higher risk of CD19 CAR T-cell failure.
Although prior inotuzumab ozogamicin (InO) has been shown to impact the fitness of collected T-cell and preinfusion antigen load, several studies have demonstrated that it can be successfully used before CD19-directed CAR T-cell therapy. High disease burden is associated with CD19 CAR T-cell failure and higher toxicities, so reducing this before infusion is strongly recommended.
Consolidative HSCT and non-HSCT approaches1
Allogeneic HSCT which is commonly used as a consolidative approach following CAR T-cell remission may decrease relapse risk; however, this may not always translate to an overall survival (OS) benefit due to the heavy pretreatment burden including a prior HSCT. Patients with early B-cell recovery (<63 days), greater disease burden, and infusion of CD19 CAR T-cells incorporating a CD28 costimulatory domain may benefit from consolidative HSCT.
Alternatives to consolidative HSCT for patients ineligible due to comorbidities, prior HSCT, or lack of an adequately matched donor include blinatumomab, InO, and maintenance-style low-dose chemotherapy. An investigation carried out at Great Ormond Street Hospital found that 3 out of 6 patients with B-cell recovery at <6 months remained alive and were disease-free after tisagenlecleucel and consolidation with maintenance-style chemotherapy.
CAR T-cell reinfusion
Reinfusion of CAR T-cells using the same construct is currently being evaluated as a relapse prevention method for patients with early B-cell recovery; however, data remain mixed. In the retrospective analysis by Myers et al.,2 reinfusions were safe, achieved durable remissions, and prolonged B-cell aplasia in 50% of patients with peripheral and bone marrow B-cell recovery.1 Conversely, an analysis by the Pediatric Real World CAR Consortium on patients reinfused with tisagenlecleucel for B-cell recovery reported that a small proportion of patients achieved B-cell aplasia with limited durable responses. Another study by Holland et al.3 reported limited efficacy and durability, as well as a significantly lower CAR T-cell expansion of several different CAR T-cell constructs.
The use of a checkpoint blockade has emerged as a potential method to prolong CAR T-cell persistence.1,4 Patients with R/R B-ALL who previously experienced CAR T-cell therapy failure achieved an enhanced clinical response and improved CAR T-cell persistence following administration of a PD-1 inhibitor after Day 14 of CAR T-cell infusion. An anti-PD1 antibody following reinfusion is currently being investigated in the CAPTiRALL study (NCT05310591).2
Enhancing CAR T-cell persistence
Optimizing the CAR T-cell design and manufacturing process can preserve T-cell stemness, prevent exhaustion, and enhance CAR T-cell persistence. Firstly, careful T-cell selection of the starting material to improve expansion and other manufacturing strategies for long-term persistence is currently being investigated. Additionally, the role of the costimulatory domains across the two different commercially approved CAR T-cell constructs is currently being considered as it relates to long-term persistence.1
To reduce the risk of immune-mediated rejection and enhance CAR T-cell persistence, several entirely human or humanized CAR T-cells are being developed. Clinical trials on humanized CD19-directed CAR T-cells in naïve patients have demonstrated complete remission (CR) rates of up to 98%. The Penn/CHOP trial (NCT02374333) reported a 12-month and 24-month relapse-free survival rate of 84% and 74%, respectively; a lower cumulative incidence of B-cell recovery (15% vs 29%) with humanized CD19-redirected CAR T-cell therapy vs tisagenlecleucel was seen in a post-hoc analysis.1
Moreover, CD19-expressing T-cell antigen-presenting cells are currently being investigated in children and young adults with R/R B-ALL at risk for early B-cell recovery (NCT03186118) to improve CAR T-cell persistence.1,5 Preliminary data showed an increase in detectable CD19 CAR T-cells in the peripheral blood of all patients after one dose suggesting secondary expansion; it also demonstrated an acceptable safety profile.1
Minimizing antigen escape
Dual-antigen targeted approaches such as coadministration, bicistronic, cotransduction, and tandem CAR T-cell therapies are being developed in B-ALL to minimize the risk of antigen escape.1,4 In a phase I trial investigating a CD19 and CD22-targeting bispecific CAR, 100% of patients with R/R B-ALL achieved an MRD-negative CR. Moreover, AUT03, an autologous transduced T-cell expressing both anti-CD19 and anti-CD22 CAR T cells resulted in a CR rate of 86%.4 Although these demonstrated comparable remission rates, their durable efficacy and dual functionality are limited.1
Another approach currently being explored to prevent antigen-positive relapse is the use of fifth-generation CAR T-cell therapies comprising a truncated interleukin-2 receptor B-chain domain with a transcription factor such as 3/5 transcription factor; these constructs have demonstrated improved cell proliferation and persistence when compared with currently available CAR T-cell therapies in preclinical models.5
Relapse management
Following CD19-directed CAR T-cell therapy relapses, the survival outcomes are poor. The Pediatric Real World CAR Consortium study reported a 12-month OS of 52%, and the CAR T-Cell Multicenter Analysis consortium study reported a 12-month OS of 49% which decreased at longer follow-up.1
There are several ways to characterize post CAR T-cell relapse, including the immunophenotype of relapsed disease, the timing of relapse, and treatment history; all of these factors can inform potential salvage strategies.1 Based on expression profile, relapses can be classified as CD19-positive relapse, CD19-negative relapse, or lineage switch.1,4 Antigen-positive relapse is generally caused by poor persistence, low potency of CAR T cells, and/or transient B-cell aplasia; whereas, antigen-negative relapse may be due to a gene mutation, alternative splicing, or lineage switch.4
Risk factors associated with CD19-positive relapse include the cumulative number of CRs as a surrogate for treatment burden. Younger age, 4-1BB costimulatory domain-containing CAR T-cells, high disease burden, and prior blinatumomab failure are associated with CD19-negative relapse. Patients with the KMT2A rearrangements have an increased prevalence of lineage switch.
Figure 2 highlights the available post CAR T-cell therapy relapse treatment options based on immunophenotype.
Figure 2. Post CAR T-cell therapy strategies based on relapse phenotype*
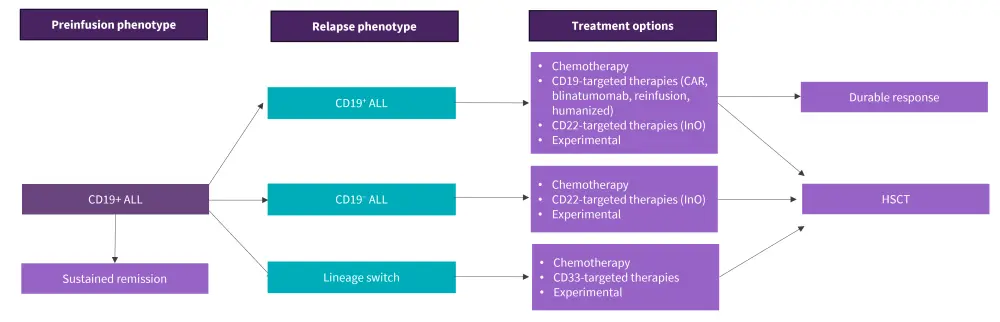
ALL, acute lymphoblastic leukemia; CAR, chimeric antigen receptor; HSCT, hematopoietic stem cell transplantation; InO, inotuzumab ozogamicin.
*Data from Lamble, et al.1
Management of CD19-positive relapse
Patients who experience CD19-positive relapse can be treated with CD19-targeting strategies such as blinatumomab, reinfusion of the previously used CD19-CAR T-cell product, or an alternative CD19-CAR T product. Blinatumomab has demonstrated higher efficacy and safety when compared with intensive chemotherapy after the first CAR T-cell relapse. Reinfusion of the previous CD19 CAR T-cell product after relapse has demonstrated CR rates of up to 50% thus it may be beneficial. CD19-targeted humanized CAR T-cells have yielded encouraging CR rates of 64% to 88% in patients with R/R B-ALL after prior CAR T. As an alternative to CD19-directed therapies, these patients may also receive CD22-targeting strategies such as InO.
Management of CD19-negative relapse
For patients with CD19-negative relapse, CD22-targeting strategies can be used, currently limited to InO and CD22-directed CAR T-cell therapies; both approaches have yielded excellent remission rates but with limited durability and the need to be followed with consolidative HSCT.
Management of lineage switch
Patients with lineage switch have poor outcomes and may require multimodality strategies such as intensive myeloid-directed therapy followed by HSCT. Gemtuzumab ozogamicin is a treatment recommended for this patient population. Moreover, with the higher incidence of KMT2A rearrangement in these patients, molecular-targeting agents such as menin inhibitors remain a promising option.
Conclusion
This review highlights the emerging prevention and management strategies for post CAR T-cell relapse, which remains a challenge in R/R B-ALL. Relapse prevention approaches currently underway include consolidative HSCT, CAR T-cell reinfusion, modifying preinfusion factors, humanized CAR T cells to enhance CAR T-cell persistence, and dual-targeted CAR T-cell therapies to minimize antigen escape. Strategies for the management of CD19-positive relapse include CD19-targeted and CD22-targeted treatments, and the management of CD19-negative relapse is limited to the use of CD22-targeted therapies. Gemtuzumab ozogamicin proves effective in addressing a heightened complexity associated with lineage switching.
Key research priorities for the prevention and management of relapse include:
- the consensus definition of B-cell aplasia and B-cell recovery;
- prospective trials on the use of blinatumomab;
- InO and maintenance-style chemotherapy post CAR T-cell therapy;
- comparison between humanized vs human CD19-directed CAR T-cell therapies on enhancing persistence;
- salvage therapies for CD19-negative relapse and lineage switch; and
- individualized sequence of salvage strategies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

