All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Progress in CAR-T therapy for T-ALL
In contrast to options for B-cell acute lymphoblastic leukemia, chimeric antigen receptor (CAR) T-cell therapy for T-cell acute lymphoblastic leukemia (T-ALL) is still in its initial stages, owing to difficulties in identifying T-ALL specific targets, T-cell aplasia and fratricide. At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, three presentations focused on the safety and efficacy of novel immunotherapeutic targets in T-ALL in the pre-clinical and clinical setting. We summarize key findings from these presentations below.
Anti CD7 CAR-T 1
Yang, et al. presented a phase I study investigating the safety and efficacy of anti-CD7 CAR T-cell therapy for patients with relapsed/refractory T-ALL.
A total of 17 patients were enrolled into this study between December 2020 and June 2020, and 14 patients were evaluated. Their baseline characteristics are summarized in Table 1.
Table 1. Patient characteristics*
|
CAR, chimeric antigen receptor; EMD, extramedullary; HSCT, hematopoietic stem cell transplant; PB, peripheral blood. |
|
|
Characteristic |
n = 14 |
|---|---|
|
Median age (range), years |
17 (3–42) |
|
Female, n |
2 |
|
Bone marrow blasts pre-CD7CAR (range), % |
11.53 (0.18–65.03) |
|
<5 |
5 |
|
5–25 |
4 |
|
>25 |
6 |
|
Patients with PB blasts pre-CD7CAR, % |
50 |
|
Median lines of prior therapies (range), n |
5 (3–8) |
|
Relapsed following Allo-HSCT, n |
3 |
|
EMD involvement, n |
5 |
|
Central nervous system leukemia |
3 |
|
Diffuse EMD |
2 |
|
Localized EMD |
3 |
|
High risk phenotype and genotypes, n |
|
|
Ph+ T-ALL |
1 |
|
ETP-ALL |
3 |
|
STIL-TAL1 |
3 |
|
RUNX1 |
2 |
All CD7CAR T-cell products were successfully manufactured, with a median transduction efficiency of 93.8% (range, 59.6–99.9%).
Two patients were given low dose (5 × 105 cells/kg), 11 were given medium dose (1–1.5 × 106 cells/kg) and one patient was given a high dose (2 × 106 cells/kg).
Efficacy
After a median follow up of 213 days, bone marrow and extramedullary disease status are summarized in Figure 1.
Figure 1. Bone marrow and EMD status following CAR T-cell infusion and consolidative allo-HSCT*
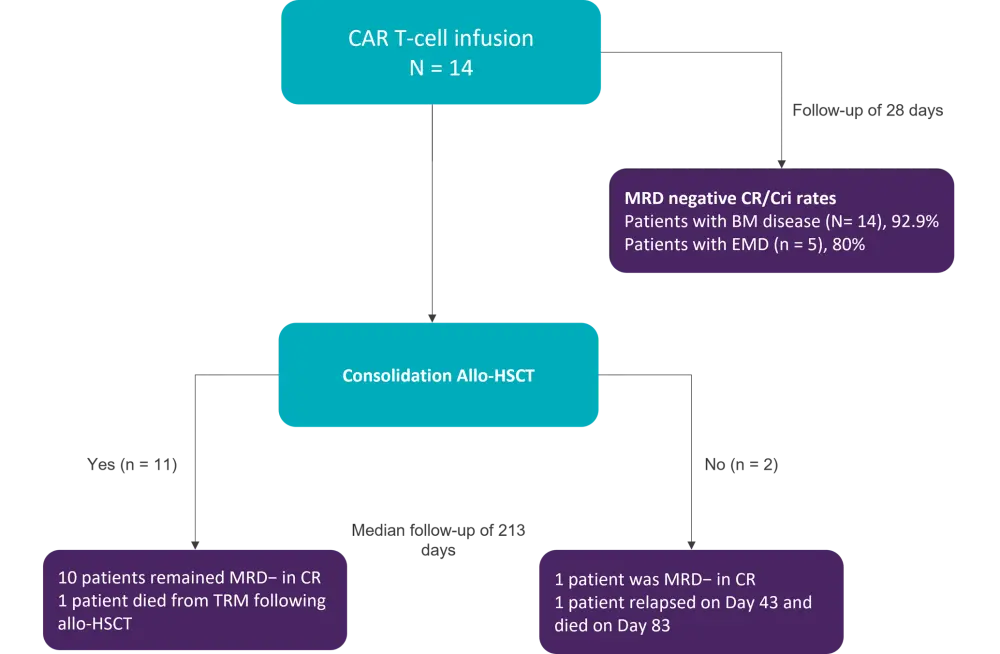
Allo-HSCT, allogeneic hematopoietic stem cell transplant; BM, bone marrow; CAR, chimeric antigen receptor; CR/CRi, complete remission/complete remission with incomplete hematologic recovery; EMD, extramedullary; MRD, measurable residual disease; TRM, transplant related mortality.
All but one patient achieved measurable residual disease negativity, irrespective of extramedullary involvement by Day 28. This patient still harbored STIL-TAL1 mutation and had lost CD7 expression. One patient died following consolidative allo-HSCT and had already received a prior allo-HSCT before CAR T-cell infusion.
Safety
A total of 13 patients experienced mild cases of cytokine release syndrome (Grade ≤2), and no patients experienced Grade >1 neurotoxicity. One case of cytomegalovirus activation was observed. For the 11 patients receiving consolidation allo-HSCT, three reported acute GvHD, and three patients had onset of chronic GvHD.
CD7CAR-T cell expansion and persistence
Using quantitative pCR, Yang reported that the median peak of CD7CAR copy number was 2.38 × 105 (range, 0.2–6.67 × 105), with a median peak at 20 days. The median time at which CD7CAR copy was persistent was 52.5 days (range, 20–120 days). Using flow cytometry, the median peak time for CD7CAR T-cell proliferation was 15 days (range, 7–21 days). The maximum proliferation proportion was 84.95% (range, 36.6–84.95%).
In summary, this phase I study demonstrated the safety and efficacy of CD7 CAR T-cell therapy in patients with heavily pretreated R/R T-ALL, inclusive of extramedullary involvement, prior allo-HSCT, and high-risk cytogenetics.
Anti-CD21 CAR-T therapy2
Maciocia, et al. presented data on the potential of CD21 as an immunotherapy target, firstly highlighting previous work which demonstrated an association of CD21 expression with T-ALL presentation and relapsed/refractory cases. In healthy blood, C21 expression was limited to B cells and a small proportion of T cells, therefore presenting an attractive target for inhibition.
Maciocia then presented a series of pre-clinical experiments observing the efficacy of anti-CD21 CAR T-cell therapy for T-ALL. The work also involved a systematic alteration of the CAR design to improve epitope binding affinity.
Proximal membrane epitope binding improves cytotoxicity
Initial anti-CD21 CARs were not adequately binding to distal epitope CD21 domains, resulting in poor immune synapse formations. As such, functional CAR-T activity was impaired, with low interferon-g and interleukin-2 (IL-2) release. To enhance the efficacy of CAR-CD21 binding, Maciocia and her team generated a library of binders for the first five sushi domains of CD21; these demonstrated improved cytotoxicity against CD21+ cells, as shown by a reduction in the percentage of malignant cells and high IFN-g release. However, background non-specific cytokine release was observed toward CD21 negative cells.
Fragment antigen binding structure improves specificity
CAR specificity was improved by re-cloning the binder into a fragment antigen-binding (Fab) structure, similar to a conventional antibody. The newly yielded NM2 Fab CARs (n = 6) showed improved in vitro function compared with NM2 cells with the original structure (n = 9), with IFN-g release much more specific to CD21-positive cell lines compared with CD21-negative cell lines.
NM2 Fab CAR transduction did not produce fratricide, with cell expansion and proliferation post transduction comparable with anti-CD19 controls and no difference in differentiation/exhaustion markers observed after 7 days of proliferation.
Specific activity of the NM2 Fab CAR was demonstrated in three low density CD21+ cell lines in vitro. Cytotoxicity and IFN-g release was significantly greater (although IL-2 release was not significantly different) for the NM2 Fab CAR than for the Ph2 Fab negative control. Moreover, the NM2 Fab CAR was able to kill low density CD21+ primary T-ALL cells from two patients, with residual cells significantly reduced compared with controls (p < 0.01 in Patient 1, p < 0.001 in Patient 2).
In vivo xenograft model
The NM2 Fab CAR was tested in a T-ALL patient-derived xenograft in vivo model in which CD21 was expressed in low copy number. The percentage of tumor in peripheral blood was significantly reduced, translating into a greater survival probability, when treated with the anti-CD21 CAR compared with an anti-CD19 CAR.
In summary, this data showed the feasibility of CD21 targeting in T-ALL and provided insight into the receptor architecture required to induce high affinity binding, with proximal membrane epitope targeting using a Fab receptor structure producing high activity and specific cytokine release.
Anti CCR9 CAR-T3
Another potential immunotherapy target, C-C motif chemokine receptor 9 (CCR9), was discussed by Maciocia, et al. Transcripts of CCR9 were previously found to be uniquely expressed in a T-ALL cell line (MOLT-4) and were confirmed by RNA-seq to be found in >70% of pediatric T-ALL cases. Previous research by Maciocia and his team showed that <5% of normal T cells expressed CCR9, and 9% of B-cells were CCR9 positive. Here, the efficacy of anti-CCR9 CAR T cells (CARCCR9) was compared with anti-CD19 CAR controls in vitro and in vivo.
In vitro testing
After 48 hours, CARCCR9 was cytotoxic in several CCR9+ (but not CCR9-) T-ALL cell lines. Additionally, the percentage of CCR9+ T-ALL targets remaining was significantly lower for CARCCR9 than for the anti-CD19 CAR (CARCD19) control. Notably, the reduction in T-ALL targets was maintained in lower density (400 copies per cell) lines.
Over 48 hours, IFN-g and IL-2 release were raised significantly with CARCCR9 in CCR9+ cell lines compared with CCR9- lines. Notably, similar levels of IFN-g and IL-2 were produced with CARCCR9 and with CARCD19 in a T-ALL cell line containing 90,902 copies of CD19 per cell and only 410 copies of CCR9.
When testing CARCCR9 against primary T-ALL samples, significant reductions in blasts were observed.
In vivo testing
CARCCR9 was tested in vivo with MOLT4 T-ALL engrafted mice engineered to express luciferase. Treatment with 8 × 105 CARCCR9 cells resulted in disease regression as evident by loss of luciferase radiance, continued weight gain, and improved survival probability past Day 20 compared to untreated and CARCD19 controls (p = 0.0032).
In a PDX T-ALL model, mice were engrafted with 8 × 105 CARCCR9 cells which significantly reduced disease burden over 124 days in the peripheral blood compared with CARCD19 and untreated controls, and again produced markedly improved survival probability (p = 0.04). Further investigation showed normal sized spleens in mice treated with CARCCR9 versus CARCD19 (p < 0.01) and untreated mice (p < 0.001), while three out of four mice had no tumor detected. The benefit of CARCCR9 was also observed in a second PDX model with even more aggressive reduction in peripheral blood tumor cells and improved survival probability over controls (p = 0.0032).
Finally, in ongoing lower density CCR9+ cell models, good disease control has so far been observed.
In summary, CCR9 shows promising potential as an immunotherapeutic target for T-ALL, with an anti-CCR9 CAR T-cell therapy producing disease regression in both in vitro and in vivo models.
Conclusion
Although in the initial stages, promising progress has been made for the use of CAR T-cell therapy in T-ALL. Efficacy has been demonstrated in a clinical setting when targeting CD7 expressing cells, and pre-clinical research has also provided insight into the feasibility of other immunotherapeutic targets, CD21 and CCR9. Alteration of the CAR design appeared to overcome non-specific CAR-T activity, which will be a key hurdle to monitor with future clinical research.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

