All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Real-world efficacy of CAR T cells for posttransplant relapse in patients with B-ALL
Do you know... Which of the following patients with R/R B-ALL can benefit from a single tisa-cel infusion?
Patients with acute lymphoblastic leukemia (ALL) who do not respond to chemotherapy and relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) have a poor prognosis. Novel cellular immunotherapies, such as CD19-directed chimeric antigen receptor (CAR) T cells, are being investigated for this high-risk patient population. The efficacy of CAR T-cell therapy has been confirmed in large studies of patients with precursor relapsed and refractory (R/R) B-cell ALL (B-ALL).
Here, we summarize a real-world retrospective study involving CAR T-cell centers in Germany that were approved to perform treatment with tisagenlecleucel (tisa-cel). The centers were asked to collect treatment data reflecting post-approval reality to verify clinical trial outcome data. In addition, the study aimed to identify patients who would benefit most from this tisa-cel treatment.1
Study design
The study was conducted between September 1, 2018, and January 1, 2022. Included patients had B-ALL, received tisa-cel, were aged ≤25 years, and had primary refractory disease, ≥2 relapses, or had relapsed after allo-HSCT.
Results
Patient characteristics
A total of 81 patients were included and the baseline characteristics were similar between patients with/without prior allo-HSCT (Table 1).
Table 1. Patient characteristics*
|
HSCT, hematopoietic stem cell transplantation; LDC, lymphodepleting chemotherapy; NA, not applicable. |
|||
|
Characteristic, % (unless otherwise stated) |
All patients |
With HSCT |
Without HSCT |
|---|---|---|---|
|
Median age (range), years |
11.5 (1–25) |
10 (1–25) |
14.5 (1.2–25) |
|
Female |
34.6 |
35.4 |
31.2 |
|
Male |
65.4 |
64.6 |
68.8 |
|
Preliminary treatment |
|||
|
Primary refractory |
1.2 |
0.0 |
6.2 |
|
Relapsed refractory |
18.5 |
0.0 |
93.8 |
|
HSCT |
80.2 |
100.0 |
NA |
|
Cytogenetics |
|||
|
Favorable |
13.6 |
10.8 |
25.0 |
|
Intermediate risk |
50.6 |
50.8 |
50.0 |
|
High risk |
35.8 |
38.5 |
25.0 |
|
Remission status at LDC |
|||
|
<5% blasts |
54.3 |
50.8 |
68.8 |
|
≥5% blasts |
45.7 |
49.2 |
31.2 |
The results showed that patients with refractory or relapsed disease, including patients who relapsed after allo-HSCT, could be rescued with a single infusion of CAR T cells.
Safety
CRS and ICANS
- Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were less frequent in the real-world study versus the ELIANA trial.
- CRS: 67.9% vs 77.3%, respectively
- ICANS: 7.4% vs 40%, respectively
- The risk of CRS was not significantly different between those who had previously had HSCT compared to those who had not (64.6% vs. 81.2%; p = 0.20).
Non-relapse events
- Two patients developed fulminant, severe CRS (Grade V) and macrophage activation that could not be controlled; the patients died 1.2 months and 0.8 months post CAR T-cell infusion.
- Two patients died of treatment-related mortality due to infection or multiorgan toxicity at 4.6 or 1.2 months after CAR T-cell infusion.
- One patient with a prior allo-HSCT followed by CAR T-cell treatment developed secondary myelodysplastic syndrome.
Efficacy
Response
- Overall, 87.8% of patients achieved complete response; however, in 9.9% of patients, 28 days of CAR T-cell infusion failed to yield a response (Figure 1).
Figure 1. Response rates at 28 days post-CAR T-cell infusion*
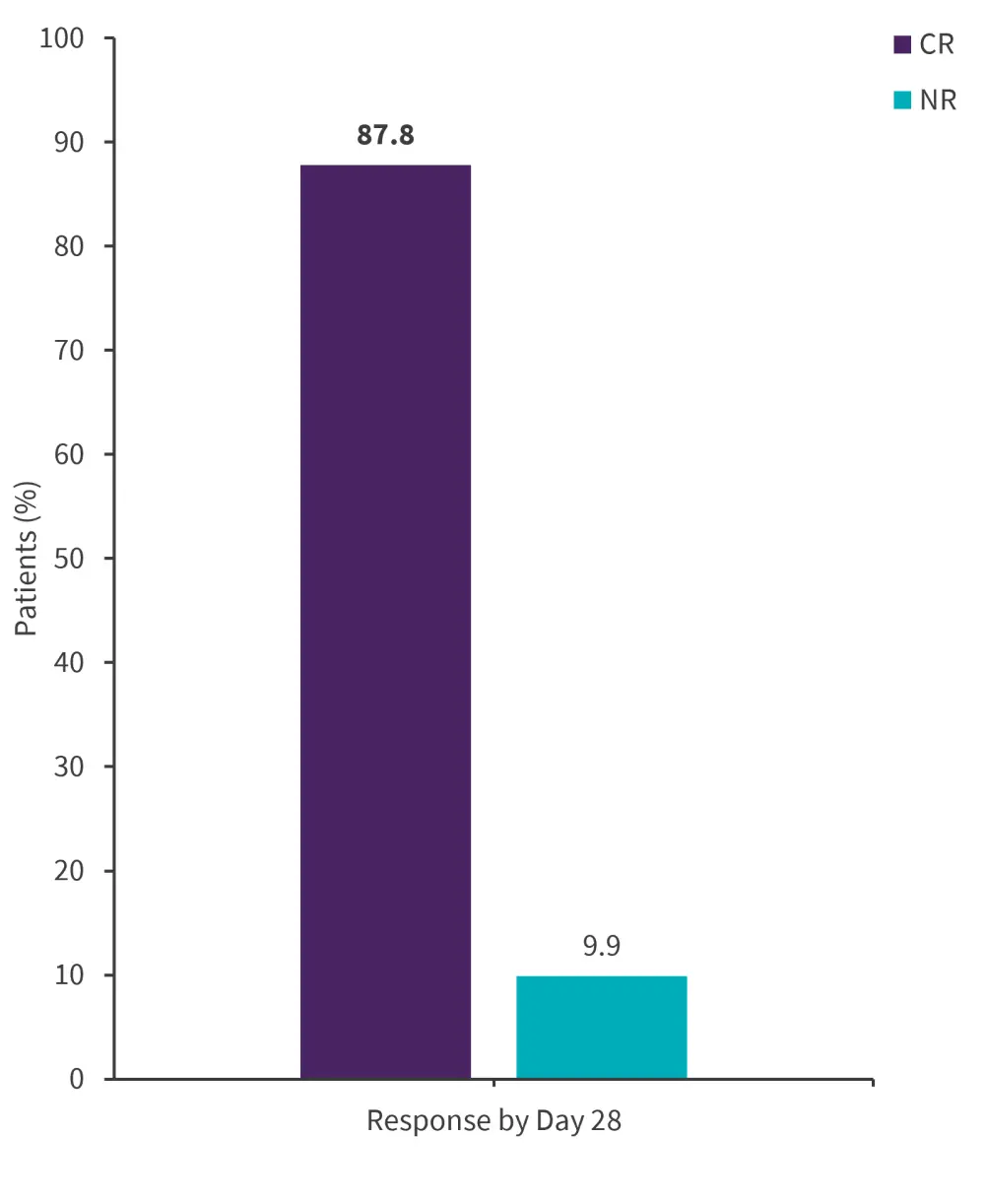
CAR, chimeric antigen receptor; CR, complete response; NR, no response.
*Adapted from Bader, et al.1
Survival
- At 2 years, the probabilities of event-free survival (pEFS), relapse-free survival (pRFS), and overall survival (pOS) were 45.3%, 51.7%, and 53.2%, respectively.
- The pEFS was not different in patients without vs with prior allo-HSCT (55.0% vs 4 3.4%, respectively).
- The analysis of potential risk factors, such as age, cytogenetics, sites of leukemia involvement, or intensity of LDC, showed that only disease burden at LDC was associated with pEFS. Patients with high-level disease (≥5%) had a pEFS of 29.6%, compared with 55.4% in patients with low-level disease (<5% blasts; p = 0.049)
Patients with previous allo-HSCT
Of the 81 patients involved in this study, 65 had received a prior allo-HSCT for high-risk or relapsed disease, and subsequently relapsed before receiving CAR T-cell therapy. With a relatively a high proportion of patients having received allo-HSCT, this was a unique population in real-world evidence and was therefore analyzed in detail.
Patients who relapsed within 6 months of receiving an allo-HSCT had a median follow-up of 28.8 months; they also had a lower pEFS compared with those relapsing more than 6 months after allo-HSCT. Furthermore, pOS and pRFS were lower in patients who relapsed within 6 months of allo-HSCT compared with patients who relapsed more than 6 months following allo-HSCT. Treatment failure was mainly due to relapse, with the cumulative incidence of relapse being higher for patients who had relapsed within 6 months of allo-HSCT compared with those who had relapsed beyond 6 months (68.5% vs 37.1%; p = 0.026). A comparison between pEFS, pOS, PRFS, and cumulative incidence of relapse is shown in Figure 2.
Figure 2. Response rates for patients who relapsed within and beyond 6 months after allo-HSCT*
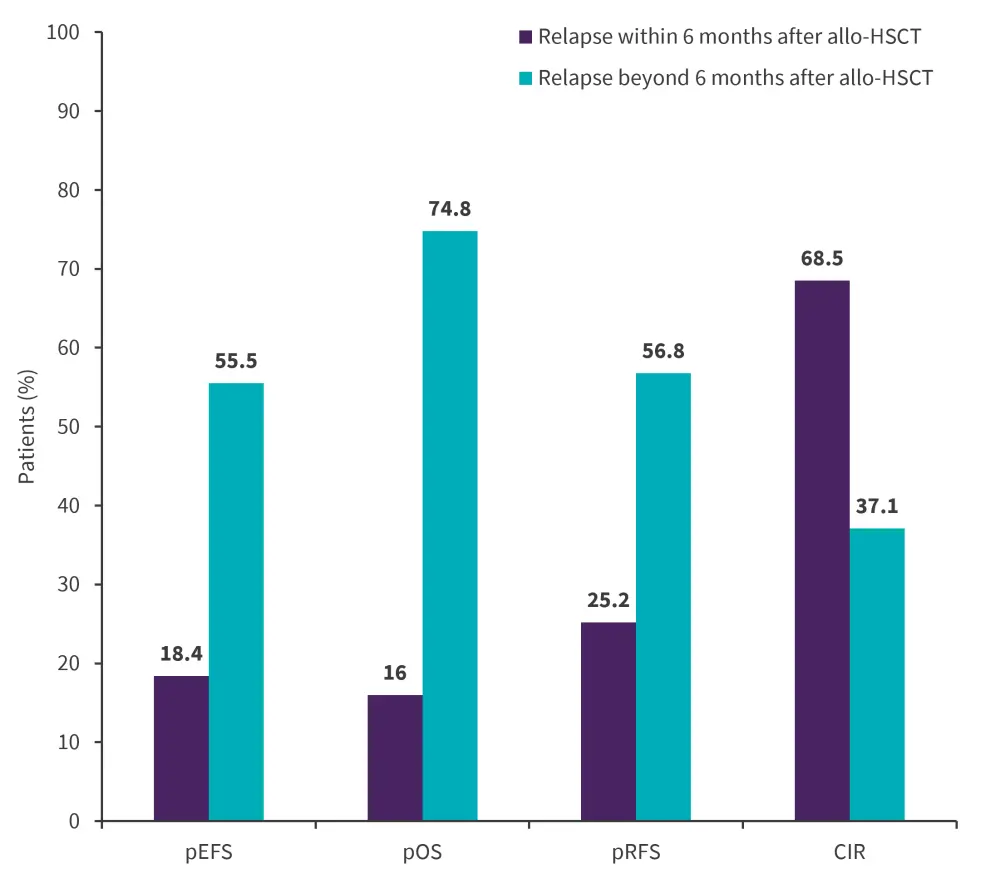
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; CIR, cumulative incidences of relapse; pEFS, probability of event-free survival; pOS, probability of overall survival; pRFS, probability of relapse-free survival.
*Adapted from Bader, et al.1
Conclusion
This real-world study demonstrated promising outcomes with tisa-cel in patients with R/R B-ALL. The results showed that patients who relapsed after allo-HSCT could be rescued with a single infusion of tisa-cel. Furthermore, patients who relapsed ≥6 months after HSCT had improved survival outcomes with a single tisa-cel infusion and no further consolidation. Prospective studies should be undertaken to further elucidate how to improve outcomes in patients who relapse during the first 6 months after allo-HSCT.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

