All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Real-world outcomes with tisagenlecleucel in CAYAs with R/R B-ALL
Relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) in children, adolescents, and young adults (CAYAs) is associated with poor prognosis.1 Tisagenlecleucel (tisa-cel) has been in commercial use for almost 5 years for the treatment of R/R B-ALL; however, available real-world data have been heterogeneous and limited across different institutions delivering tisa-cel.1
Liora M. Schultz et al.1 performed a retrospective, multi-institutional study across 15 institutions in the United States to collect data from tisa-cel experience to demonstrate real-world outcomes and influencing factors. The results of this study have been recently published in the Journal of Clinical Oncology, and we are pleased to provide a summary of key points.
Study design
A national consortium was established to allow cross-institutional reporting and analysis among 15 pediatric centers.
- Intent-to-treat (ITT) response analysis included 200 patients who underwent leukapheresis and cell shipment for planned standard-of-care tisa-cel regardless of receiving the treatment (Figure 1).
- All patients who received tisa-cel (including through an expanded managed access protocol or individual investigational new drug approval) were evaluated for response, toxicity, and survival with a minimum follow-up of 28 days.
- Primary objective: Overall complete response (CR) rate at Day 28 following tisa-cel, measured by bone marrow (BM) and/or peripheral blood morphology and minimal residual disease
- Secondary objectives: Overall survival (OS) and event-free survival (EFS) at 6 and 12 months following tisa-cel
- Exploratory objective: Risk factors for response and survival
Figure 1. Patient disposition*
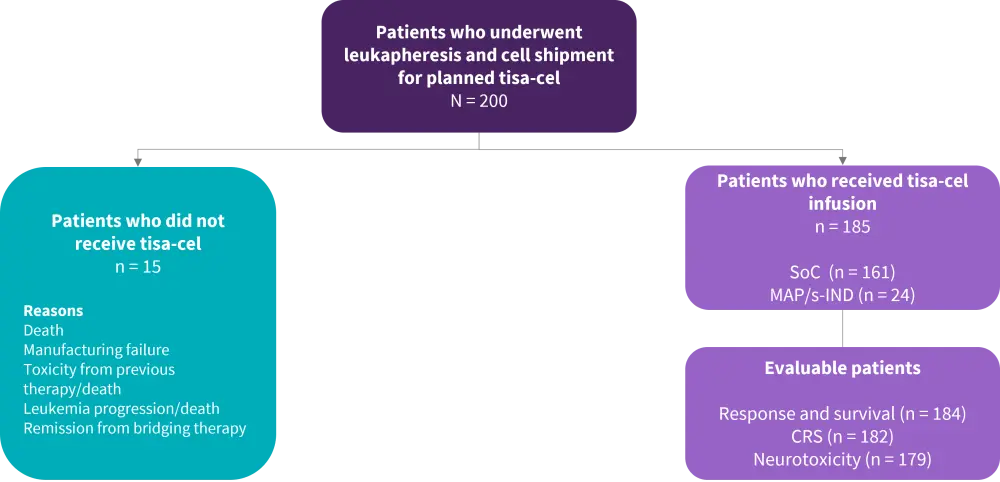
CRS, cytokine release syndrome; MAP, managed access protocol; s-IND, single-patient investigational new drug; SoC, standard of care; tisa-cel, tisagenlecleucel.
*Adapted from Schultz, et al.1
Results
Of 200 patients, 185 received tisa-cel infusion; the median age at infusion was 12 years (range, 0–26 years). The median follow-up from tisa-cel therapy was 335 days (range, 6–863 days). The median number of prior therapy lines was three (range, 1–10), with a median duration of 33 months (range, 3–171 months). Most patients (83%) were treated for relapsed disease. Table 1 summarizes patient characteristics by infused and noninfused cohorts.
Table 1. Patient characteristics (N = 200)*
|
allo-HSCT, allogeneic hematopoietic stem cell transplantation; CAR, chimeric antigen receptor; MPAL, mixed phenotype acute leukemia. *Adapted from Schultz, et al.1 |
||
|
Characteristic, % |
Infused |
Noninfused |
|---|---|---|
|
Sex |
||
|
Male |
60 |
60 |
|
Female |
40 |
40 |
|
Cytogenetics |
||
|
High risk |
36 |
27 |
|
Ph-positive (p190 and p210) |
5 |
0 |
|
Ph-like |
13 |
13 |
|
MPAL |
1 |
0 |
|
Intermediate risk |
26 |
20 |
|
Low risk |
13 |
13 |
|
Unknown |
25 |
40 |
|
Prior lines of therapy, % |
||
|
1–3 |
61 |
33 |
|
4–5 |
29 |
33 |
|
>5 |
10 |
33 |
|
Prior allo-HSCT |
35 |
33 |
|
Prior CD19 therapy |
||
|
Blinatumumab |
18 |
40 |
|
CD19 CAR |
3 |
0 |
|
Prior CD22 therapy |
||
|
Inotuzumab |
17 |
53 |
|
CD22 CAR |
2 |
0 |
|
Disease status |
||
|
Refractory without relapse |
16 |
7 |
|
1 relapse |
37 |
40 |
|
≥2 relapses |
46 |
40 |
Efficacy
The Day 28 CR rate was 79% (95% confidence interval [CI], 72–84) in the ITT population (excluding patients who did not receive tisa-cel due to CR from prior therapy; n = 197). Efficacy results from 184 infused evaluable patients are summarized in Table 2.
Table 2. Results from infused evaluable patients*
|
CI, confidence interval; CR, complete response; DoR, duration of response; EFS, event-free survival; MAP, managed access protocol; OS, overall survival; s-IND, single-patient investigational new drug. |
|
|
Response, % (95% CI) |
|
|---|---|
|
Overall Day 28 CR rate (n = 184) |
85 (79–89) |
|
Patients who received MAP/s-IND |
83 |
|
Patients with detectable disease prior to tisa-cel infusion (n = 134) |
81 (73–86) |
|
DoR at 6 months |
75 |
|
DoR at 12 months |
62 |
|
MRD negativity by flow cytometry in pts with morphologic CR (n = 153) |
97 |
|
Survival outcomes |
|
|
Median OS |
Not reached |
|
6-month OS, % |
85 |
|
12-month OS, % |
72 |
|
6-month EFS, % |
62 |
|
12-month EFS, % |
50 |
Among responders:
- 37% relapsed (median time from infusion to relapse: 101 days, range, 30–645)
- 28% underwent post-CAR HSCT (median time from infusion to HSCT: 199 days, range 36–565)
- 20 patients were in remission at the time of HSCT, while 19 patients proceeded to transplant following relapse post-tisa-cel.
In multivariate analysis, high burden disease (defined by ≥5% BM lymphoblasts, any circulating lymphoblasts and/or CNS3, or non-CNS extramedullary disease) was found to be significantly associated with a lower morphologic CR rate (73% vs 98% in low burden disease and 100% in undetectable disease; p < 0.0001). OS, EFS, and DoR at 6 and 12 months were also lower with high burden disease: 12-month OS was 58% (vs 85% in low burden disease and 95% in undetectable disease; p < 0.0001) and 12-month EFS was 31% (vs 70% in low burden disease and 72% in undetectable disease; p < 0.0001). Young age (3–10 years) at diagnosis (p = 0.006) and increased time from diagnosis to infusion (p < 0.001) were associated with improved OS.
Safety
Of 185 patients, 183 were evaluable for safety at Day 28 (incomplete data, n = 2). The results of safety analysis are provided in Table 3.
Table 3. Safety analysis*
|
AE, adverse event; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; PICU, pediatric intensive care unit; TLS, tumor lysis syndrome. *Adapted from Schultz, et al.1 |
|
|
AE |
n, % |
|---|---|
|
CRS (N = 183) |
|
|
Any grade, % |
63 |
|
Grade ≥3, % |
21 |
|
Median CRS onset time, days (range) |
5 (0–14) |
|
Median duration of CRS, days (range) |
4 (1–42) |
|
Neurotoxicity (evaluable, n = 179) |
|
|
Any grade, % |
21 |
|
Grade ≥3, % |
7 |
|
Cerebral edema, % |
0.6 |
|
Cerebral hemorrhage, % |
0.6 |
|
Median neurotoxicity onset time, days (range) |
6 (3–25) |
|
Median duration of neurotoxicity, days (range) |
5 (1–203) |
|
TLS (n = 175) |
|
|
Yes, % |
7 |
|
Grade 4 neutropenia persistent >28 days (evaluable, n = 147) |
|
|
Yes, % |
16 |
|
Infections (evaluable, n = 181) |
|
|
≥1 infection, % |
40 |
|
Median onset, days (range) |
68 days (1–559) |
|
CAR-related hospitalizations (overall cohort, n = 184) |
|
|
Inpatient care, % |
82 |
|
Median duration, days (range) |
14.5 (1–75) |
|
PICU, % |
31 |
|
Median duration, days (range) |
6 (1–33) |
A total of 51 patients died after tisa-cel infusion.
- Five patients died before Day 28, due to either leukemia, infection, cytokine release syndrome (CRS) or neurotoxicity.
- Other causes of death included infection, transplant complications and cardiac events.
- Non-relapse mortality was 7%.
In the univariate analysis, high disease burden was associated with increased CRS severity and higher rate of pediatric intensive care unit-level care compared with low burden disease and undetectable disease (p < 0.0001 for both). Relapse versus refractory status was also associated with increased CRS.
Conclusion
This study indicates that response and survival outcomes of tisa-cel in the real-world setting are similar to that seen in previous studies. Importantly, high disease burden is shown to be associated with inferior outcomes, suggesting high-risk disease may benefit from additional interventional approaches to optimize outcomes of CAR T-cell therapy.
Limitations of this study arise from its retrospective nature, differences in reporting across centers, and changes in definitions of disease response and relapse.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

