All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Results from the COG AALL0331 trial in a low-risk subset of children with standard-risk B-ALL
For pediatric patients with B-cell acute lymphoblastic leukemia (B-ALL), a risk-adapted therapy intensification has led to survival improvements, with rates of 5-year survival exceeding 90%.
In 2004, the Children’s Oncology Group (COG) revised the ALL risk-based classification system, identifying a subset of patients with standard-risk (SR) B-ALL: SR-low. SR-low patients had no extramedullary disease, rapid early response to induction therapy, end-induction bone marrow (BM) minimal residual disease (MRD) <0.1%, and one of two favorable cytogenetic features (either ETV6-RUNX1 fusion or trisomies of chromosomes 4, 10, and 17).
Given the efficacy and tolerability of pegaspargase intensification in younger patients, the COG AALL0331 trial (NCT00103285) sought to investigate whether post-induction pegaspargase intensification on a low-intensity treatment backbone would improve the continuous complete remission (CCR) rate in a SR-low subset of patients with B-ALL.1
Study design
The study schema is shown in Figure 1. Between 2005 and 2010, 5,377 patients with SR B-ALL aged 1─9 years and with an initial white blood cells (WBC) count <50,000/μL were enrolled in the study. Of these, 5,303 received a three-drug induction with dexamethasone, vincristine, and pegaspargase, plus intrathecal chemotherapy with cytarabine and methotrexate. Following the induction, risk assignment was refined into SR-low, SR-average, and SR-high, and 1,857 patients met the eligibility criteria for the inclusion in the SR-low cohort.
The low-risk criteria included:
- No corticosteroid pretreatment
- No central nervous system, testicular, or other extramedullary leukemia
- Favorable cytogenetics
- No high-risk features
- Rapid early response to induction therapy (<5% BM blasts by Day 15, and end-induction BM MRD <0.1%)
Patients in the SR-low cohort were then randomized 1:1 to receive standard consolidation and interim maintenance with (low-risk with intensified pegaspargase [LRA]; n = 928) or without (low-risk standard [LRS]; n = 929) four additional pegaspargase doses administered every 3 weeks.
After September 2008, during interim maintenance, all patients received the escalating dose of intravenous (IV) methotrexate without leucovorin rescue (LRS with IV methotrexate [LRS-IV]; LRA with IV methotrexate [LRA-IV]) instead of oral methotrexate. After June 2008, during delayed intensification, all patients received alternate-week dexamethasone instead of continuous dexamethasone. A summary of the treatment schedule is shown in Figure 2.
The primary endpoint of the study was CCR.
Figure 1. Study schema*
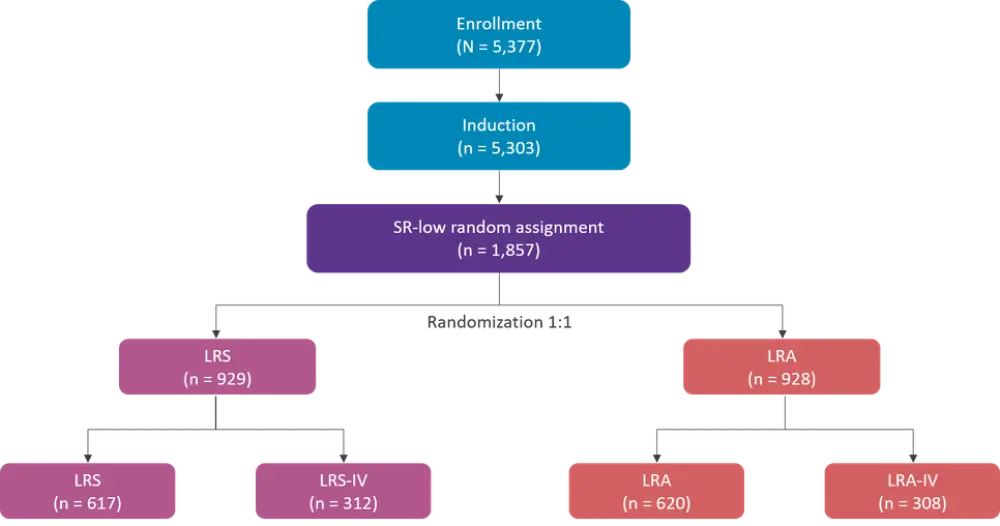
IV, intravenous; LRA, low-risk with intensified pegaspargase; LRS, low-risk standard; SR, standard-risk.
*Data from Mattano et al.1
Figure 2. Treatment summary*
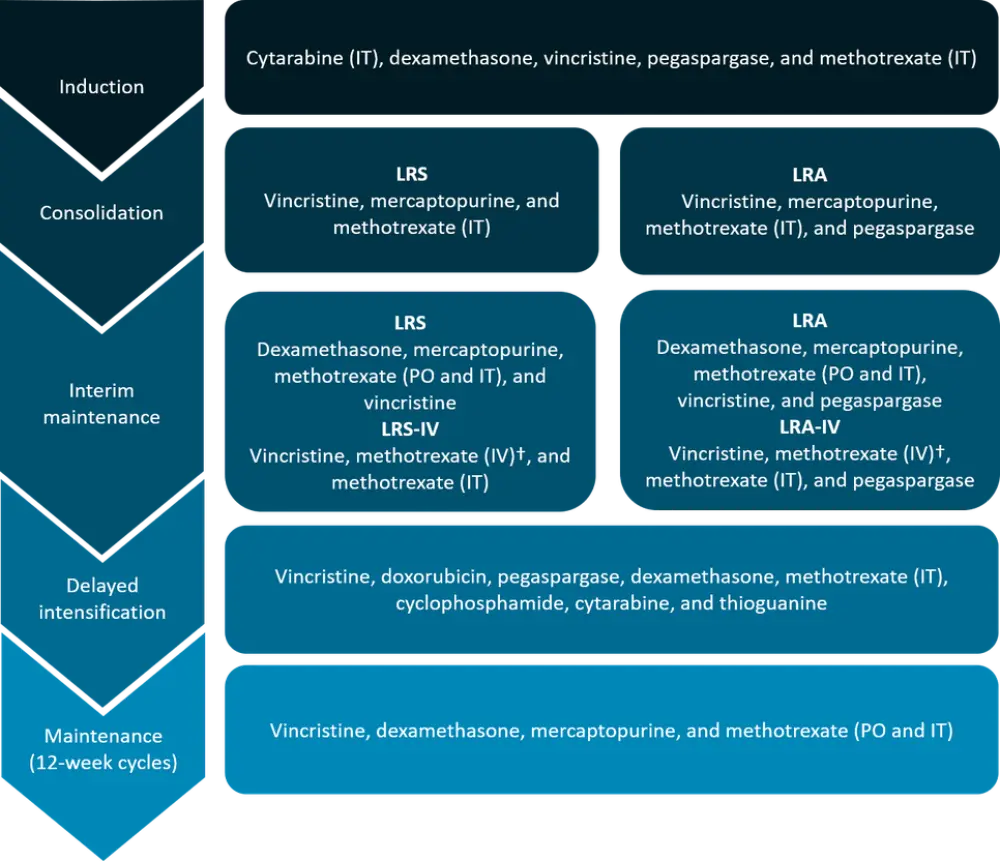
IT, intrathecal; IV, intravenous; LRA, low-risk with intensified pegaspargase; LRA-IV, LRA with IV methotrexate; LRS, low-risk standard; LRS-IV, LRS with IV methotrexate; PO, oral.
*Adapted from Mattano et al.1
†Dose escalated by 50 mg/m2 every 10 days for a total of five doses, adjusted for toxicity.
Patient characteristics
Characteristics of the patients in the SR-low cohort are reported in Table 1.
Table 1. Patient characteristics*
|
|
LRS |
LRA |
|---|---|---|
|
LRA, low-risk with intensified pegaspargase; LRS, low-risk standard; MRD, minimal residual disease; WBC, white blood cell. |
||
|
Median age at diagnosis, years (range) |
3.83 (1.00─9.95) |
3.85 (1.02─9.92) |
|
Median WBC × 1,000/μL (range) |
6.90 (0.30─49.54) |
6.60 (0.40─49.40) |
|
Cytogenetics, n (%) |
|
|
|
Marrow induction Day 29, n (%) |
|
|
|
MRD induction Day 29, n (%) |
n = 917 |
n = 918 |
Outcomes
Outcomes are reported in Table 2. No significant differences in either CCR rates or OS were observed between LRA/LRA-IV and LRS/LRS-IV regimens, but a superior CCR was observed in the LRA arm when considering patients assigned to pre-amendment regimens (LRA and LRS). In addition, superior CCR and OS were observed in patients with triple trisomy compared with ETV6-RUNX1 fusion, and superior CCR was observed in patients with <5% BM blasts at Day 8 compared with those not achieving <5% BM blasts until Day 15.
Table 2. 6-year CCR and OS for the SR-low cohort*
|
|
6-year CCR |
6-year OS |
|---|---|---|
|
BM, bone marrow; CCR, continuous complete remission; LRA, low risk with intensified pegaspargase; LRS, low risk standard; MRD, minimal residual disease; OS, overall survival |
||
|
All SR-low patients |
94.7% ± 0.6% |
98.7% ± 0.3% |
|
LRA and LRA-IV |
95.3% ± 0.8% |
98.1% ± 0.5% |
|
LRA |
95.3% ± 0.9% |
97.9% ± 0.6% |
|
LRA-IV |
95.4% ± 1.3% |
98.6% ± 0.7% |
|
LRS-IV |
96.3% ± 1.2% |
99.7% ± 0.4% |
|
Triple trisomy |
96.3% ± 0.8% |
99.4% ± 0.3% |
|
Entire SR-low cohort |
|
|
|
Day 8 PB MRD data available (n = 1,036) |
|
|
In a comparative analysis with the SR-average cohort (n = 422; patients treated with therapy identical to LRS or LRS-IV, who met all the SR-low criteria but without favorable cytogenetics), SR‑low versus SR-average patients showed:
- Superior CCR, 94.0% ± 0.8% vs 88.6% ± 1.7% (HR, 1.95; 95% CI, 1.34─2.85; p = 0.0004)
- Superior OS, 99.2% ± 0.3% vs 96.1% ± 1.0% (HR, 5.42; 95% CI, 2.37─12.39; p = 0.0001)
Factors associated with inferior outcome in the SR-average vs SR-low cohort were:
- Age, ≥6 vs <6 years (HR, 1.821; 95% CI, 1.197─2.771; p = 0.0051)
- Risk group, SR-average vs SR-low (HR, 2.598; 95% CI, 1.448─4.662; p = 0.0014)
- Day 29 MRD, ≥0.01% vs <0.01% (HR, 2.706; 95% CI, 1.727─4.239; p < 0.0001)
- Cytogenetics, ETV6-RUNX1 fusion vs triple trisomies (HR, 1.807; 95% CI, 1.000─3.264; p = 0.0498).
Safety
A summary of the disease-free survival events and toxicities is reported in Table 3.
Table 3. Disease-free survival events and toxicities*
|
|
LRS |
LRA |
|---|---|---|
|
DFS, disease-free survival; LRA, low risk with intensified pegaspargase; LRS, low risk standard; SMN, second malignant neoplasm. |
||
|
DFS event, n |
|
|
|
Targeted toxicity |
||
|
Post-induction, n (%) |
n = 927 |
n = 923 |
|
Nontargeted Toxicity |
||
|
Consolidation, n (%) |
n = 927 |
n = 923 |
|
Interim maintenance, n (%) |
n = 919 |
n = 876 |
|
Consolidation, interim maintenance, delayed intensification, n (%) |
n = 927 |
n = 923 |
Conclusions
- Patients with SR-low B-ALL treated with low-intensity post-induction therapy with or without intensified pegaspargase had 6-year CCR 94.7% and OS 98.7%
- No significant differences in CCR and OS were observed between the two arms of the study
- Favorable cytogenetics was associated with superior CCR and OS, with triple trisomy associated with better CCR and OS compared with ETV6-RUNX1 fusion
- Remission deaths and the most common targeted toxicities, pancreatitis and osteonecrosis, were more frequent with intensified pegaspargase therapy
Altogether, results from this study show that, in selected settings, a reduction of treatment intensity that can limit toxicity without compromising the outcome may be successful. Also, the standard therapy without intensified pegaspargase could be easily delivered in an outpatient setting, which has important implications for ALL treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

