All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Survival and prognostic factors of AYA patients with ALL receiving a modified pediatric BFM-90 regimen
Adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL) are still a difficult-to-treat patient population. High mortality (80% of ALL deaths occur within this patient subset) and lack of representation in clinical research highlights a need to better understand optimal treatment approaches and relevant prognostic factors for this age group.1 Previous literature has illustrated superior overall survival (OS) in AYA patients when using pediatric protocols compared with adult regimens. Nevertheless, further research on pediatric regimens is required, with more concise AYA age definition, greater representation, and toxicity data. Previously, the ALL Hub published a study comparing a pediatric and an adult therapy regimen in AYA patients with ALL. An overview on the main differences in pediatric and AYA treatment approaches is summarized on the ALL Hub here.
For prognosis, measurable residual disease (MRD) has previously demonstrated to be effective for predicting survival outcomes. However, the optimal time for MRD assessment, to assist treatment decisions as well as prognostic assessment, is still in debate. Listen also to our podcast with Janine Stutterheim discussing the value of MRD in infants with ALL.
A single-center, retrospective analysis was recently published by Akhil Rajendra and colleagues in Blood Advances, assessing the efficacy and toxicity of a modified pediatric regimen, Berlin-Frankfurt-Münster 90 (BFM-90), in a defined and well-represented AYA group (aged 15–25). The authors also investigated the association of different baseline disease characteristics, including MRD, with OS and event-free survival (EFS).1 The key findings are summarized below.
Study design
- The study design of this retrospective study, conducted in AYA patients treated at the Tata Memorial Centre in India between 2013 and 2016, is summarized in Figure 1.
- The median follow-up was 41 months.
- Primary endpoints: 3-year OS and EFS.
- Secondary endpoints: Association of MRD and other high-risk factors (white blood cell count, cytogenetics, hypodiploidy, infections, and central nervous system [CNS] stage) with EFS and OS.
- MRD assessment was performed post-induction phase 1a, and for MRD+ patients, this was repeated post phase-1b, post protocol M, and post reinduction (see Figure 1).
- Safety data: Infections and treatment-related toxicities that led to either death, discontinuation, or a change of protocol.
Figure 1. Study design for the BFM-90 protocol and drugs given during each stage*
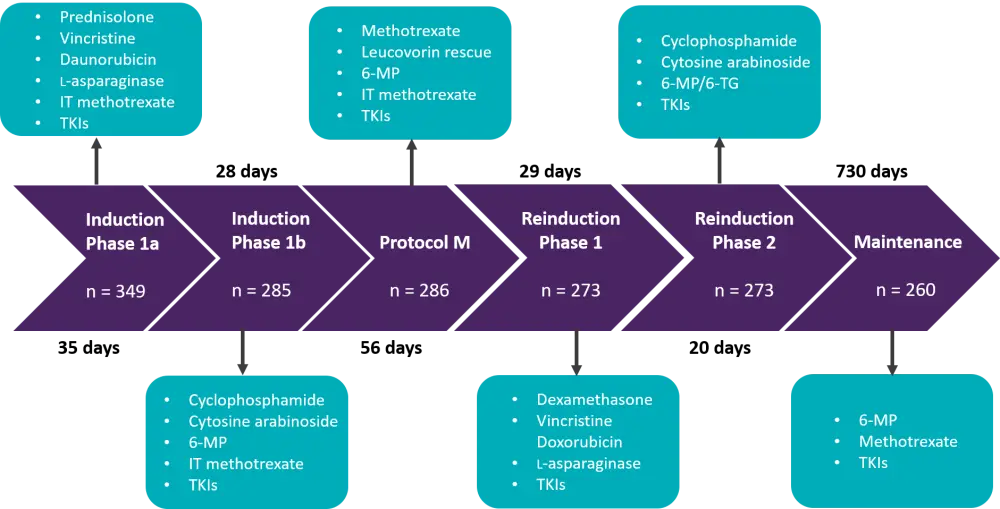
TKI, tyrosine kinase inhibitors (imatinib or dasatinib); 6-MP, 6-mercaptopurine; 6-TG, 6-thioguanine; IT, intrathecal.
*Data from Rajendra et al.1
Baseline characteristics
A total of 463 patients with ALL, aged 15–25 years, were enrolled. Of these, 349 received the BFM-90 protocol. Baseline patient characteristics are presented in Table 1.
Table 1. Baseline patient characteristics*
|
ALL, acute lymphoblastic leukemia; T-ALL; T-cell ALL; B-ALL, B-cell ALL; CNS, central nervous system; ETP, early T-cell precursor ALL; WBC, white blood cell. |
|
|
Characteristic |
Patients |
|---|---|
|
Median age, years (range) |
18 (15–25) |
|
Sex (male), % |
78.2 |
|
Diagnosis, % |
|
|
CNS involvement, % |
5.7 |
|
High-risk, %† |
34 |
|
Hypodiploidy, % |
10.3 |
|
High-risk WBC baseline count, % |
|
Key results
Primary endpoints
3-year OS and EFS with the BFM-90 protocol were 61.8% and 59.4%, respectively.
- Patients with high-risk ETP/near-ETP had significantly inferior 3-year OS and EFS vs patients with non-ETP ALL (OS, 32.5% vs 71.3%, p < 0.0001; EFS, 30.4% vs 69.4%, p < 0.0001).
- No significant difference in OS or EFS was found between the standard-risk and high-risk groups.
Secondary endpoints
A univariate cox-regression analysis was performed for the association of diagnosis (T-ALL, B-ALL), baseline CNS involvement, MRD positivity post induction, MRD positivity beyond induction, WBC-based risk, cytogenetic risk, and infections with EFS and OS.
- Only baseline CNS involvement (CNS stage 2 or 3), postinduction MRD positivity, positive MRD status beyond induction, and development of infections were associated with inferior EFS and OS.
A multivariate analysis was performed for baseline CNS involvement, MRD positivity post induction, MRD positivity beyond induction, and infections.
- MRD status post induction was the only prognostic factor significantly associated with survival outcomes.
- The 3-year EFS and OS rates of MRD− patients vs MRD+ patients were 77.4% vs 41% and 79.2% vs 44.8%, respectively.
- For MRD− compared with MRD+ patients post induction, EFS had an odds ratio of 2.400 (95% CI, 1.324–4.352; p = 0.004) and the odds ratio of OS was 2.583 (95% CI, 1.592–4.193; p < 0.001).
Also of note, following the median 41-month follow-up, 79 (22.6%) patients had relapsed and 18 (23%) were classified as having experienced CNS relapse. Only 15 (4.3%) patients underwent allogeneic hematopoietic stem cell transplantation.
Safety
- 164 patients experienced infections (all grade) throughout treatment, 40 of which led to treatment discontinuation or death (24.4%).
- The total number of deaths by the end of induction was 21 (6.5%) and 19 were attributable to infections.
- Most infections leading to discontinuation or death were in the induction phase (77.5%).
- The most common infection episodes were fungal (35%) and bacterial (30.6%).
- The total number of noninfectious Grade 2–5 adverse events was 297.
- There was a total of 102 treatment related toxicities, and the most common episodes were seizures (12.8%), followed by cortical venous thrombosis (6.3%), pancreatitis (4.8%), hyperglycemia (2.5%), posterior reversible encephalopathy syndrome (1.4%), and avascular necrosis (1.1%).
Conclusion
This retrospective study demonstrates the feasibility and safety of using the BFM-90 protocol for a well-defined AYA cohort with ALL. Although rates of toxicity were low, the authors highlight a need for preventative measures against fungal and bacterial infections during induction. Furthermore, the data provides further evidence for the association of postinduction MRD negativity with superior survival outcomes, while patients with high-risk ETP ALL were identified has having inferior survival outcomes.
Limitations outlined by the research team were that the study was neither risk- or response-adapted, it was a single-center experience, and newer, high-risk molecular factors that may influence survival outcomes were excluded.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

