All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
The prognostic impact of genetic alterations and MRD status in KMT2A-rearranged B-ALL
KMT2A-rearranged (KMT2A-r) B-cell precursor acute lymphoblastic leukemia (B-ALL) is considered a high-risk ALL subtype in both children and adult patients. Patients with this ALL subtype have traditionally experienced poor outcomes, with a lack of robust data on adult patients treated with modern protocols and a general uncertainty as to the optimal treatment strategy for this patient population. Recent efforts have indicated that the presence of certain genetic co-mutations and minimal residual disease (MRD) status may provide an accurate means of predicting clinical outcomes and guiding treatment decisions for patients.
Kim et al.1 recently analyzed a large cohort of patients treated in three consecutive clinical trials, published in Blood, to identify subsets of patients with heterogenous clinical outcomes. Below, we summarize their key findings.
Study design
- The study included patients with newly diagnosed Philadelphia-negative B-ALL.
- Patients were treated in three consecutive clinical trials (NCT00222027, NCT00327678, and NCT02617004) by the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL), where they underwent modern intensive protocols.
- The presence of genetic rearrangements was determined via cytogenetic analysis, and MRD was assessed via quantitative polymerase chain reaction (qPCR).
- The study objectives were to investigate the association between genetic subtypes and clinical outcomes (CIR, DFS, and OS )
Key findings
- Overall, 1,091 patients aged 15-59 years with newly diagnosed Philadelphia-negative B-ALL were included in the study (KMT2A-r positive: n = 141).
- KMT2A-r B-ALL was shown to be associated with typical high-risk presenting features including older age, increased white blood cell counts, and pro-B phenotype; however, this did not appear to influence the ability to achieve complete response.
- Historical risk criteria could not predict distinct clinical outcomes within KMT2A-r B-ALL.
- Genetic analysis showed KMT2A-r B-ALL to be associated with a specific pattern of genetic alterations, primarily targeting the RTK-RAS pathway, and the TP53, CDKN2A, and IKZF1 tumor-suppressor genes.
- TP53 alterations and IKZF1 deletions were associated with a significantly worse cumulative incidence of relapse (CIR; p = 0.005 and p = 0.01, respectively), disease-free survival (p = 0.034 and p = 0.053, respectively), and overall survival (p = 0.007 and p = 0.11, respectively).
- MRD1 and MRD2 positivity among patients with KMT2A-r B-ALL were significantly associated with worse outcomes.
- Further analysis demonstrated an advantage in response evaluation and predicting treatment outcomes for gKMT2A-based MRD over IG/TR-based MRD.
- Combining oncogenetics and gKMT2A-based MRD allowed for the highlighting of three subgroups of patients with distinct clinical outcomes (Figure 1): Onco–/MRD–, Onco–/MRD+, and Onco+/MRD+.
Figure 1. Patient outcomes*
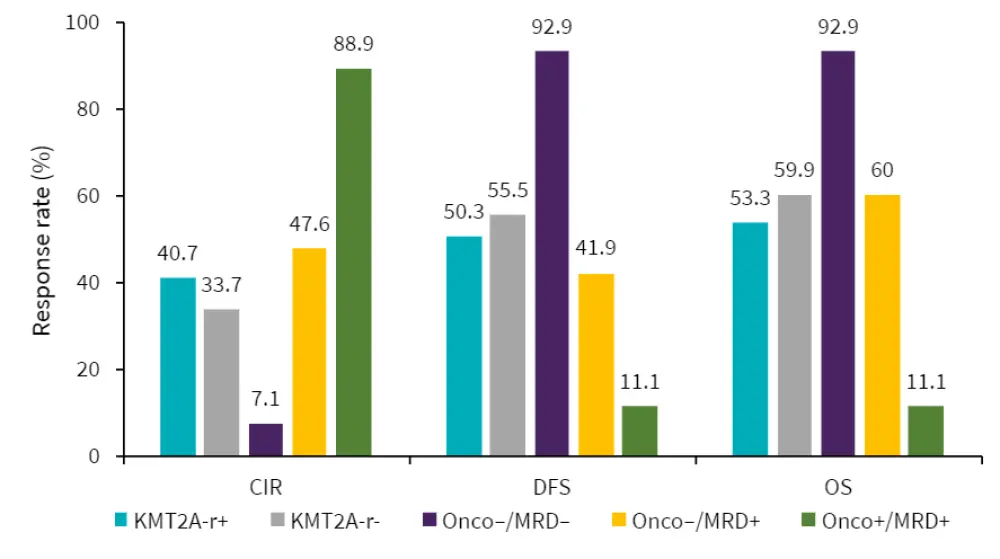
CIR, cumulative incidence of relapse; DFS, disease-free survival; MRD, minimal residual disease; Onco, oncogenetics; OS, overall survival.
*Adapted from Kim et al.1
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

