All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Tisagenlecleucel in pediatric and young adult patients with Down syndrome-associated relapsed/refractory acute lymphoblastic leukemia
Do you know... In a post-hoc analysis of three clinical studies, evaluating tisagenlecleucel in 16 patients with DS-ALL, what was the best overall response at 3 months?
Introduction
Individuals with Down syndrome (DS) are 20 times more vulnerable to developing leukemia. Patients with DS-associated acute lymphoblastic leukemia (DS-ALL) are at an increased risk of relapse, treatment-related toxicities, infections, and mortality when compared with patients who have ALL without DS; demonstrating an urgent unmet need for effective but less toxic treatment options in this population.
The ALL Hub has previously reported on minimal residual disease and survival outcomes in patients with DS and ALL. Below, we summarize the key findings of a post hoc analysis by Laetsch, et al., published in Leukemia, investigating the safety and efficacy of tisagenlecleucel in a subgroup of patients with DS-ALL enrolled in the ELIANA, ENSIGN, and B2001X studies.1
Study design
Patients with DS-ALL aged 5–22 years and treated with tisagenlecleucel were included in the post hoc analysis. A total of 16 patients with DS-ALL were included from two phase II trials (ELIANA, ENSIGN), and a phase IIIb managed access protocol (B2001X) (Figure 1). One prospective patient with DS-ALL in the ELIANA trial underwent leukapheresis and exhibited successful manufacturing but died due to disease progression prior to receiving tisagenlecleucel infusion.
Endpoints
- Efficacy endpoints included responses at Day 28, Month 3, and Month 6 post tisagenlecleucel infusion, best overall response, duration of remission, relapse-free survival, overall survival, and time-to-event endpoints.
- Safety endpoints included all adverse events.
Figure 1. Patients with DS-ALL were treated with tisagenlecleucel in three clinical studies*
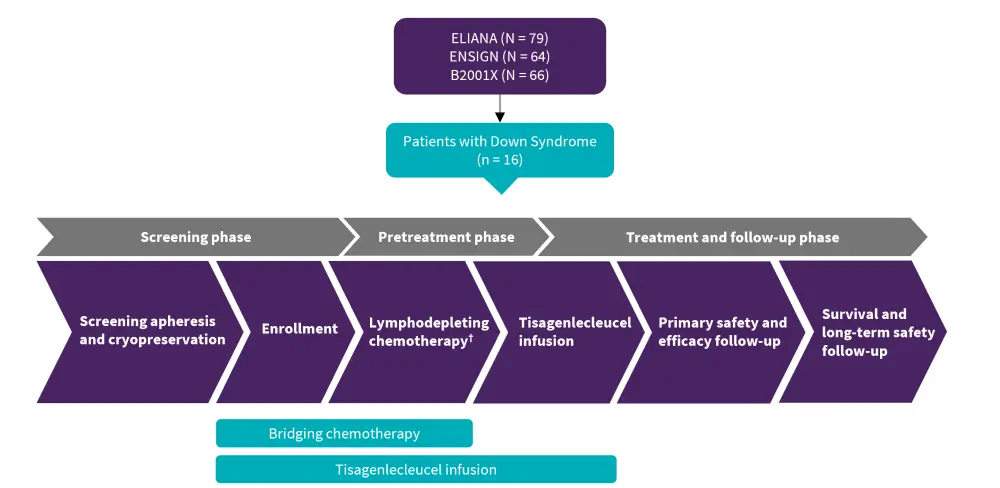
DS-ALL, Down syndrome-associated acute lymphoblastic leukemia.
†Fludarabine (30 mg/m2 IV daily for four doses) and cyclophosphamide (500 mg/m2 IV daily for two doses, starting with the first dose of fludarabine) was planned to end 2–14 days prior to tisagenlecleucel infusion.
*Adapted from Laetsch, et al.1
Results
The baseline characteristics for patients included in this study are shown in Table 1. Overall, 16 patients with DS-ALL were included in the analysis.
Table 1. Demographics and baseline clinical characteristics of patients with DS-ALL*
|
ALL, acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CNS, central nervous system; CRS, cytokine release syndrome; DS-ALL, Down syndrome-associated ALL; SCT, stem cell transplant. |
|
|
Characteristics, % (unless otherwise stated) |
Patients with DS-ALL (n = 16) |
|---|---|
|
Median age (range), years |
8.5 (5.0–22.0) |
|
Male |
69 |
|
Prior SCT |
25 |
|
Previous lines of therapy, n (%) |
|
|
Median (range) |
2 (1–4) |
|
1 |
5 (31) |
|
2 |
5 (31) |
|
3 |
3 (19) |
|
4 |
3 (19) |
|
Median morphologic blast count in bone marrow (range) |
42.0 (3.0–96.4) |
|
Disease status |
|
|
Refractory |
6 |
|
Relapsed |
94 |
|
Median time from the last relapse to CAR T-cell infusion (range), months |
3.1 (1–11) |
|
CNS status classification at enrollment |
|
|
CNS-1 |
81 |
|
CNS-2 |
19 |
|
CNS-3 |
0 |
Efficacy
- The median follow-up was 13.2 months. The ORR within 3 months of infusion was 88% (Figure 2).
Figure 2. Response rates (Month 3) in patients with DS-ALL treated with tisagenlecleucel*
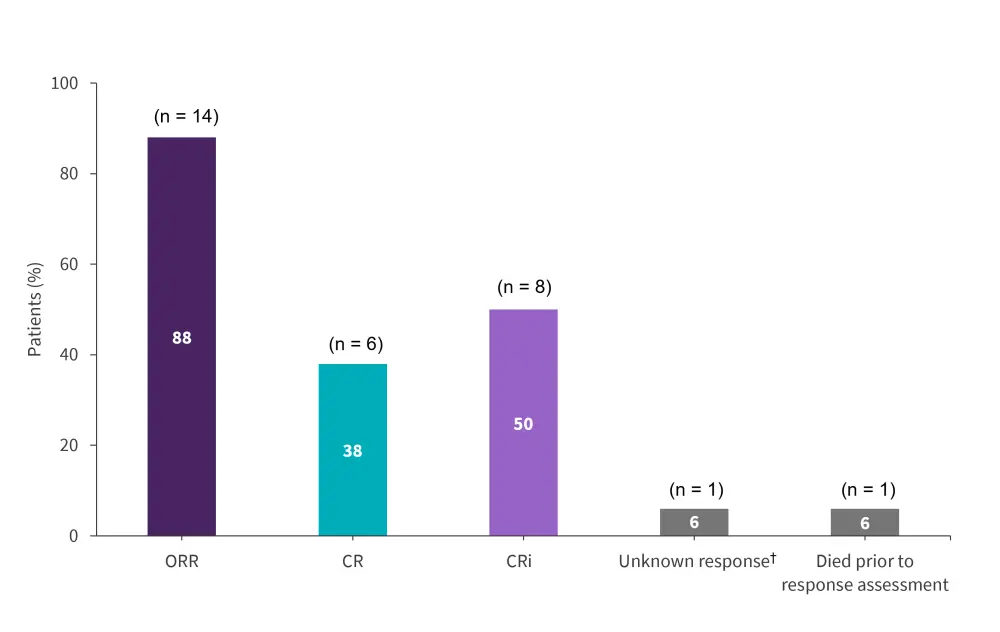
CR, complete remission, CRi, complete response with incomplete blood count recovery; ORR, overall remission rate.
*Adapted from Laetsch, et al.1
†Response assessment was not performed within the protocol-required time frame and the patient died before further assessment.
- Overall, 14 (88%) patients achieved a best overall response of CR or CRi within 3 months, 12 (86%) patients achieved minimal residual disease-negative status.
- All 14 patients who achieved remission had achieved CR or CRi by Day 28.
- The median duration of remission was 22.7 months, and the probability of remaining in remission at 6 months, 1-, 2-, and 3-years post-infusion was 69%, 57%, 38%, and 38%, respectively. The probability of overall survival at 6 months, 1-, 2-, and 3-years post-infusion was 94%, 86%, 75%, and 60%, respectively.
- Six (43%) patients relapsed after CR (three, CD19−; three, unknown), between 80–721 days post-infusion.
- Ongoing remissions in nine patients ranged from 6–48 months.
- Median overall survival was not reached.
Safety
- All 16 patients with DS-ALL achieved comparable safety outcomes compared with a pooled cohort of patients without DS from the pivotal ELIANA study.
- Any-grade and Grade 3/4 adverse events occurred in 16 and 14 patients, respectively; most patients reported cytokine release syndrome and cytopenias (Figure 3). The course and management of cytokine release syndrome in the DS-ALL cohort were similar to the pooled cohort of patients without DS.
- Tisagenlecleucel expansion and long-term persistence were consistent with previous reports.
- High-grade (Grade 3) infections occurred >8 weeks post-infusion, some occurring in the context of ongoing cytopenias; however, no infection-related deaths were reported.
- No cardiac serious adverse events were reported.
Figure 3. Selected AEs occurring within 8 weeks of tisagenlecleucel*
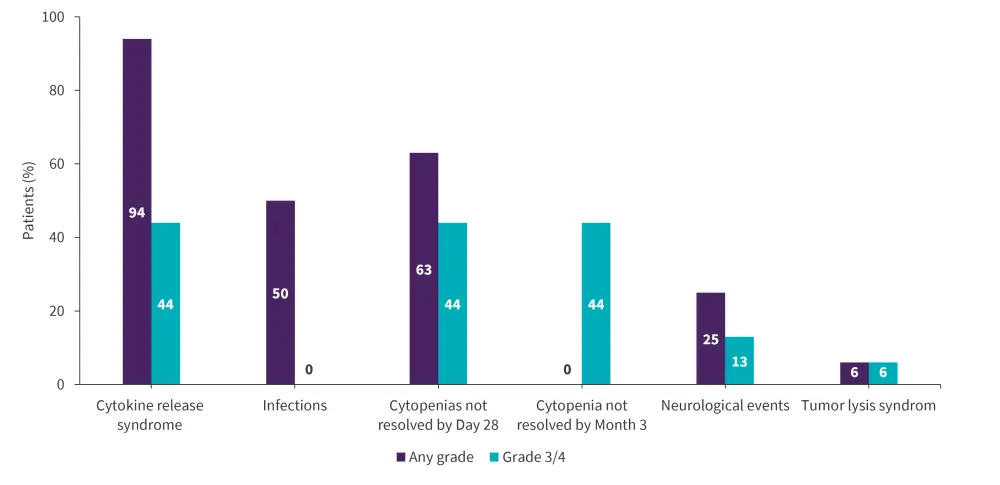
AE, adverse event.
*Adapted from Laetsch, et al.1
Conclusion
This post hoc analysis showed the promising efficacy and safety outcomes of tisagenlecleucel in pediatric/young adult patients with relapsed/refractory B-cell ALL and DS. High remission rates, an acceptable safety profile, and promising long-term outcomes are encouraging signs for pediatric/young adult patients with DS-ALL, a population with an unmet need for more efficacious and less toxic treatment options. The findings of this post hoc analysis are preliminary and further data is required to confirm the clinical benefit of tisagenlecleucel in this setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

