All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Venetoclax and navitoclax for R/R ALL and lymphoblastic lymphoma
Despite the development of novel therapeutic and chemotherapeutic agents, a great percentage of patients with relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL) respond badly to treatment and have poor prognosis.1 Effective anti-leukemic activity in ALL xenografts seems to be achieved when venetoclax, a highly selective B-cell lymphoma-2 (BCL-2) inhibitor, is combined with the anti-BCL-2 and anti-BCL-extra-large (BCL-XL) inhibitor, navitoclax.2 These findings indicate that a dual BCL-2 and BCL-XL inhibition might provide a new treatment combination for patients with R/R ALL or LL.
During the 2020 European Hematology Association (EHA) Annual Congress, our ALL Hub Steering Committee member, Elias Jabbour, presented results from a phase I trial (NCT03181126) investigating the combination of venetoclax and navitoclax for pediatric and adult R/R ALL and LL. We hereby provide a summary of these results.
Study design
- Multicenter, open-label, dose-escalation, phase I clinical trial
- Eligible patients ≥ 4 years old with measurable R/R ALL or radiographically confirmed R/R LL
- Refractoriness was defined as persistent disease after at least two courses of chemotherapy
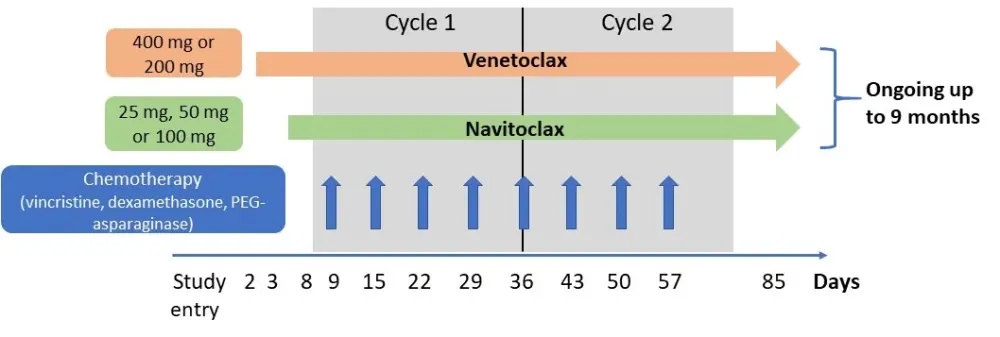
- The study design and dosing schedules are depicted below in Figure 1. Briefly, patients received 400 mg of venetoclax or a weight-adjusted equivalent (200 mg) once daily, and oral navitoclax daily at three dose levels (25, 50, 100 mg) for patients ≥ 45kg or two dose levels (25, 50 mg) for patients < 45 kg
- Patients could receive chemotherapy with vincristine, dexamethasone, and PEG-asparaginase at the investigator’s discretion
- Primary endpoint was the safety and pharmacokinetics of venetoclax and navitoclax
- Secondary endpoints included complete response (CR) rate, progression-free survival, overall survival, and the proportion of patients proceeding to stem cell transplantation or chimeric antigen receptor (CAR) T-cell therapy
Figure 1. Study design of the phase I trial investigating venetoclax plus navitoclax in R/R ALL and LL2
Results
Patient characteristics
- Patient baseline characteristics are shown below in Table 1. In general, patients were heavily pretreated with many having failed prior cellular and immune therapies like CAR T-cell therapy, blinatumomab or inotuzumab ozogamicin (anti-CD22)
Table 1. Patient baseline characteristics2
|
ALL, acute lymphoblastic leukemia; BM, bone marrow; CAR T, chimeric antigen receptor T-cell therapy; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LL, lymphoblastic lymphoma; SCT, stem cell transplantation |
|
|
Baseline characteristic |
All patients (N = 47) |
|---|---|
|
Median age (range), years Pediatric (< 18 years old) |
29 (6–72) 26% |
|
Males, % |
62 |
|
Type of primary cancer, % B-ALL T-ALL LL |
53 40 6 |
|
ECOG PS, % 1 2 |
72 14 |
|
Baseline BM blasts Median (range) ALL patients with < 5% |
52% (0–99) 11% |
|
Median number of prior lines (range) Had prior blinatumomab, % Had prior inotuzumab ozogamicin, % Had prior CAR T, % Had prior SCT, % |
4 (1–10) 28 15
13 28 |
|
Median time since last therapy, months (range) |
2 (0.1–21) |
|
Grade 3/4 thrombocytopenia, % |
43 |
Safety
- Ten fatal treatment-emergent adverse events (TEAEs) were reported but only one was considered as related to the drug combination (intestinal ischemia)
- Of the 30 patients who died, 87% had a relapse
- The most common Grade 3–4 TEAEs reported with venetoclax and navitoclax are shown below in Table 2
- In total, 13% of patients discontinued the venetoclax/navitoclax combination due to treatment related toxicity
- Two patients developed tumor lysis syndrome after ≥ 1 dose of venetoclax which was managed and resolved
Table 2. Most common Grade 3–4 TEAEs2
|
ALT, alanine aminotransferase; TEAEs, treatment-emergent adverse events |
|||
|
TEAEs (N = 47) |
Grade 3–4 (≥ 15% of patients), % |
Possible association to venetoclax/navitoclax, % |
|
|---|---|---|---|
|
Any |
98 |
74 |
|
|
Hematological |
|||
|
Febrile neutropenia |
47 |
19 |
|
|
Neutropenia |
28 |
21 |
|
|
Anemia |
17 |
9 |
|
|
Thrombocytopenia |
26 |
21 |
|
|
Non-hematological |
|||
|
Hypokalemia |
23 |
2 |
|
|
ALT increase |
19 |
4 |
|
|
Sepsis |
19 |
4 |
|
|
Blood bilirubin increase |
15 |
4 |
|
|
Pneumonia |
15 |
2 |
|
- With regards to dose-limiting toxicities (DLTs) induced by navitoclax, one DLT was reported in 16 patients at Dose Level 1 (25 mg), two DLTs in 11 patients at Dose Level 2 (25 mg, 50 mg), and five DLTs in 20 patients in Dose Level 3
- Thus, the recommended phase II dose for navitoclax in combination with the 400 mg of venetoclax was established at 50 mg for patients ≥ 45 kg and at 25 mg for patients < 45kg
- Since the main DLT observed across all navitoclax dose levels was delayed count recovery, a safety expansion cohort (n = 22) is currently evaluating a 21-day dosing schedule instead of 28-day, to help with blood count recovery
Efficacy
- With regards to preliminary efficacy data, these are very promising and are shown below in Table 3
- CR rates were ≥ 50% across patient subgroups, including those R/R to:
- Blinatumomab (62%)
- Inotuzumab ozogamicin (57%)
- SCT (63%)
- CAR T-cell therapy (50%)
- Following B-ALL subtype analysis (21 patients classified into 11 subgroups), the authors reported that responses were across all subtypes in patients with B-ALL, while T-ALL subtype analysis is ongoing
- Treatment was discontinued in 46 patients: Most patients either relapsed (n = 20) or proceeded to SCT or CAR T-cell therapy (n = 13)
- ALL or LL patient cell samples were assessed for BCL-2 and BCL-XL dependency via BH3 profiling
- B-ALL patients at baseline had more diverse BCL-2 and BCL-XL dependency when compared to T-ALL patients
- In T-ALL patients, there was a time-dependent shift towards a BCL-XL or a mix of BCL-2/ BCL-XL dependency
Table 3. Preliminary efficacy of navitoclax plus venetoclax in R/R ALL and LL patients2
|
ALL, acute lymphoblastic leukemia; BM, bone marrow; CAR T, chimeric antigen receptor T-cell therapy; CR, complete response; CRi, CR with incomplete count recovery; CRp, CR with incomplete platelet recovery; DoR, duration of response; MRD, measurable residual disease; LL, lymphoblastic lymphoma; NE, not evaluable; NR, not reached; OS, overall survival; PR, partial response; SCT, stem cell transplantation |
||||
|
Response |
B-ALL (n = 25) |
T-ALL (n = 19) |
LL (n = 3) |
All patients (N = 47) |
|---|---|---|---|---|
|
CR, CRi, CRp, % ALL patients with ≥ 5% BM blasts, % ALL patients with morphologic CR at baseline, % |
64 65 NE |
53 50 75 |
67 — — |
60 59 75 |
|
PR, % |
12 |
0 |
0 |
6 |
|
MRD-negative CR, CRi, CRp in ALL, % |
56 |
60 |
— |
58 |
|
Median DoR, (95% CI), months |
9.1 (1.4–14.6) |
4.2 (0.8–12.3) |
NE (NE–NE) |
4.2 (2.3–11.5) |
|
Median OS (95% CI), months |
9.7 (4.0–15.7) |
6.6 (3.2–12.5) |
NR (2.0–NE) |
7.8 (4.0–12.5) |
|
Proceeded to SCT or CAR T, % |
32 |
16 |
67 |
28 |
Conclusion
The results of this phase I trial indicate that the combination of venetoclax and low-dose navitoclax is well tolerated in patients with R/R ALL or LL, at the recommended phase II dose of 400 mg of venetoclax and 25/50 mg of navitoclax (weight-dependent). Prolonged cytopenia is the most relevant safety concern with this treatment combination. The preliminary efficacy data are very promising especially due to the heavily pretreated nature of this patient population with high rates of MRD negative complete remissions. A clinical follow-up with biomarker analysis, as well as a safety expansion cohort with 21-day treatment cycles to manage the observed prolonged blood count recoveries, are currently ongoing.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

