All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
A predictive prognostic scoring system based on residual disease in ALL
Most patients with acute lymphoblastic leukaemia (ALL) respond well to induction chemotherapy, with >80% of adult patients achieving a hematologic complete remission (CR).1 However, after relapse there is a low likelihood of remission with chemotherapy, and allogeneic haematopoietic stem cell transplantation (allo-HSCT) is therefore performed as one of the curative treatment options for refractory or relapsed (R/R) ALL, particularly for patients who do not achieve a CR.
The outcomes for patients transplanted in non-CR status have not yet been clearly determined. Henceforth, there is a need to identify subgroups that will benefit most from transplant and to optimize pretransplant therapeutic strategies. To fill this gap in knowledge, a multicentre retrospective cohort study for ALL patients transplanted with non-CR status was carried out by Nakamura, et al. to correlate patient characteristics with posttransplant prognosis.1
Study design
In total, 663 R/R ALL patients who were ≥16 years of age and underwent allo-HSCT at non-CR status were enrolled.
The prognostic scoring system was developed and validated by
- determining the pre-HSCT patient characteristics that were statistically associated with overall survival (OS) by multivariate analysis,
- assigning risk scores to each significant variable based on hazard ratios; and,
- assessing whether event-free survival (EFS), relapse rate, and non-relapse mortality (NRM) could be stratified by risk score.
Results
Pre-HSCT characteristics for the entire population of non-CR ALL patients are given in Table 1.
Table 1. Patient characteristics*
|
BM, bone marrow; CsA, cyclosporine A; GvHD, graft versus host disease; HCT-CI, hematopoietic cell transplantation specific comorbidity index; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; MAC, Myeloablative conditioning; MMF, mycophenolate mofetil; MTX, methotrexate; Ph, Philadelphia chromosome; PIF, primary induction failure; PS, performance status; RIC, reduced-intensity conditioning; TAC, tacrolimus; TBI, total body irradiation; WBC, white blood cell. |
|
|
Characteristic, % (unless otherwise stated) |
N = 663 |
|---|---|
|
Median age (range), years |
38 (16–74) |
|
Male |
57.6 |
|
Performance Status |
|
|
0 or 1 |
82.2 |
|
≥2 |
17.8 |
|
HCT-CI |
|
|
0–2 |
85.1 |
|
≥3 |
14.9 |
|
Median WBC count (range), ×109/L |
17.1 (0–1157) |
|
Immunophenotype |
|
|
B-lineage |
76.0 |
|
T-lineage |
20.4 |
|
Others |
3.6 |
|
Ph positive |
21.0 |
|
Extramedullary disease positive |
22.8 |
|
Median BM blast counts at HSCT (range) |
13.2 (0–100) |
|
Disease status at HSCT |
|
|
PIF |
30.6 |
|
Relapse |
69.4 |
|
HSCT related factors |
|
|
MAC |
70.9 |
|
RIC |
29.1 |
|
Full TBI |
63.3 |
|
Graft type |
|
|
Bone marrow |
39.5 |
|
Peripheral blood |
28.7 |
|
Cord blood |
31.8 |
|
GvHD prophylaxis |
|
|
TAC + MTX |
44.0 |
|
CsA + MTX |
24.6 |
|
TAC + MMF |
11.9 |
|
Others |
19.5 |
|
Donor-recipient HLA disparity |
|
|
HLA matched |
39.2 |
|
HLA mismatched |
60.8 |
Outcomes after allo-HSCT in the whole population of non-CR patients at two years were as follows:
- Overall survival (OS) and event free survival (EFS) were 31.1% (95% confidence interval [CI], 27.6–35) and 22.3% (95% CI, 19.1–25.7), respectively.
- Complete remission after HSCT was achieved in 74.8% patients.
- The cumulative incidence of hematologic relapse (including HSCT refractory patients) was 55.9% (95% CI, 51.9–59.7) and the non-relapse mortality (NRM) was 21.8% (95% CI, 18.7–25.1).
Shorter OS was significantly associated with older patient age, poorer performance status, higher hematopoietic cell transplantation specific comorbidity index, higher bone marrow (BM) blast count at HSCT, and relapsed disease status as identified by multivariate analysis (Table 2). The BM blast count was the most significant risk factor for OS in the whole-cohort analyses of non-CR ALL patients.
Table 2. Multivariate analysis for OS*
|
BM, bone marrow; CI, confidence interval; HCT-CI, hematopoietic cell transplantation specific comorbidity index; HR, Hazard ratio; HSCT, hematopoietic stem cell transplantation. |
|||
|
Variable |
HR |
95% CI |
p value |
|---|---|---|---|
|
Age at HSCT, years |
|||
|
30–49 |
1.24 |
0.99–1.55 |
0.056 |
|
≥50 |
1.48 |
1.18–1.86 |
<0.001 |
|
Performance Status ≥2 |
1.86 |
1.49–2.33 |
<0.001 |
|
HCT-CI score ≥3 |
1.38 |
1.07–1.76 |
<0.001 |
|
BM blast count at HSCT, % |
|||
|
10–49 |
1.72 |
1.36–2.16 |
<0.001 |
|
≥50 |
1.96 |
1.58–2.44 |
<0.001 |
|
Relapsed disease at HSCT |
1.5 |
1.21–1.84 |
<0.001 |
Subgroup analyses
Subgroup analyses according to pre-HSCT variables were carried out to identify the influence of individual factors on transplant outcomes. Lower BM blast count (<10%) was associated with significantly superior OS in nearly all the subgroups, compared with medium BM blast count (10%–49%) and higher BM blast count (≥50%). Therefore, it was suggested that lower blast count can be used as a predicting factor for superior OS and universal indicator for better patient prognosis. Superior OS was observed in the primary induction failure (PIF) group when compared with the relapsed group. OS in the PIF group was derived from both suppressed relapse and NRM (Figure 1).
Figure 1. Transplant outcomes for patients with PIF vs patients in relapse at time of HSCT*
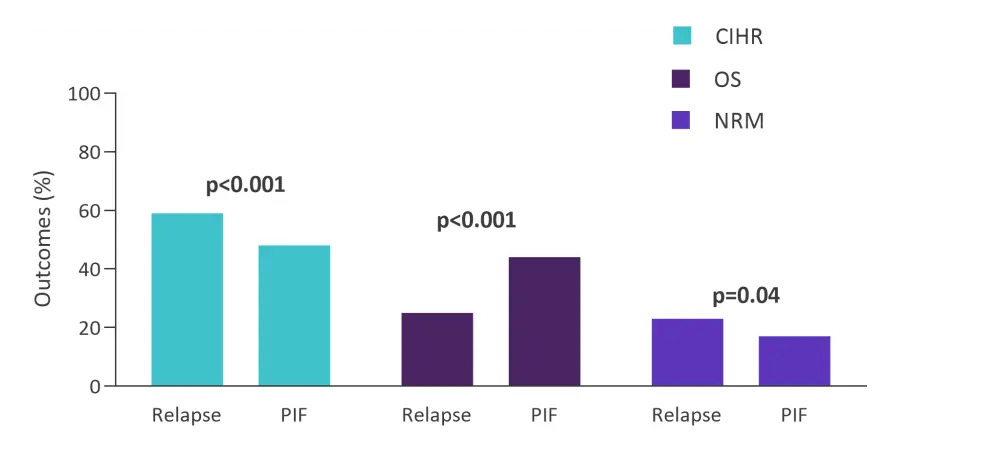
CIHR, cumulative incidence of hematologic relapse; HSCT, hematopoietic stem cell transplantation, NRM, non-relapse mortality; OS, overall survival; PIF, primary induction failure.
*Adapted from Nakamura, et al.1
The prognostic scoring system
The prognostic scoring system was developed based on five covariates identified on multivariate analysis; the HRs for OS were used to calculate the scores for each covariate (Table 3). The total score ranged from 0 to 11 with a median score of 4. Patients in the worst score category (≥5, n = 316) showed the worst prognosis (OS at two years, 15.6%), whereas those with the best score category (0–2, n = 139) demonstrated significantly superior outcomes (OS at two years, 59.5%). EFS, relapse, and NRM were clearly stratified with the risk score.
Table 3. Predictive scoring system for prognosis risk in non-CR ALL*
|
BM, bone marrow; HCT-CI, hematopoietic cell transplantation specific comorbidity index; HSCT, hematopoietic stem cell transplantation. |
|
|
Variable |
Point |
|---|---|
|
Age |
|
|
30–49 years |
1 |
|
≥50 years |
2 |
|
Performance Status ≥2 |
3 |
|
HCT-CI ≥3 |
1 |
|
BM blast counts at HSCT |
|
|
10%–49% |
2 |
|
≥50% or more |
3 |
|
Relapsed disease at HSCT |
2 |
Conclusion
The total OS data reported in this study showed that patients with R/R ALL who were transplanted when they had not achieved a CR had suboptimal prognosis. To determine whether prognosis was improved in certain subgroups of patients, a prognostic scoring system was developed using combinations of five major risk factors relating to inferior OS.
Among the five risk factors, a low BM blast count showed the most significant association with improved prognosis, both in the whole cohort and in all the subgroups. Therefore, a pre-HSCT treatment strategy to reduce tumor burden should be considered a priority.
It was established that the group with the lowest risk score (≤2) had a remarkably good prognosis with a lower incidence of relapse and NRM. These predictive analyses indicate that the patients in this subgroup can overcome the disease even if they undergo transplantation without achieving CR. Thus, the proposed scoring system can be used as a tool to predict posttransplant prognosis for allo-HSCT patients as well as to decide the treatment strategy before allo-HSCT.
Furthermore, patients who underwent HSCT at PIF had a better prognosis than those transplanted at relapse. A shorter time from diagnosis to transplantation in the PIF group (median, 5.7 months vs 11.2 months) may have resulted in less exposure to toxic chemotherapeutic regimens and contributed to a lower NRM (17.8% vs 23.6%). Thus, the optimal timing for HSCT in R/R ALL patients should be investigated further.
Study limitations included lack of information about pretransplant chemotherapies and pre-HSCT remission duration, and absence of external validation for the established prognostic scoring system.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

