All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Ponatinib, chemotherapy, and transplant in adults with Ph+ ALL: results from the phase II PONALFIL trial
Introduction
Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) is a subtype of ALL characterized by the presence of the BCR-ABL1 rearrangement. It is the most common genetic subtype of ALL in adult patients, occurring in 25–30% of young adult patients and 40–50% of older adult and elderly patients. Outcomes for this patient subgroup have historically been poor; however, the introduction of tyrosine kinase inhibitors (TKI) in combination with traditional chemotherapy approaches followed by allogeneic hematopoietic stem cell transplant (alloHSCT) has improved outcomes. The addition of third-generation TKIs, such as ponatinib, has been shown to further improve outcomes. Below, we provide a summary of the key safety and efficacy data from a phase II, open-label, PONALFIL clinical trial (NCT02776605) of ponatinib, standard induction and consolidation chemotherapy followed by alloHSCT in adult patients with newly diagnosed Ph+ ALL, published by Ribera, et al., in Blood Advances in 2022.1
Study design
Eligible patients were aged 18–60 years with newly diagnosed Ph+ ALL
- Eastern Cooperative Oncology Group (ECOG) Performance Status ≤2;
- normal cardiac function as defined by an ejection fraction of ≥50%; and
- adequate organ function as defined by serum bilirubin ≤3 mg/dL and serum creatinine ≤3 mg/dL.
Patients underwent a 7-day steroid pretreatment cycle during which the presence of the BCR-ABL1 transcript was confirmed. This was followed by induction chemotherapy for 4 weeks and consolidation chemotherapy for 2 months (Figure 1).
Figure 1. Treatment schedule*
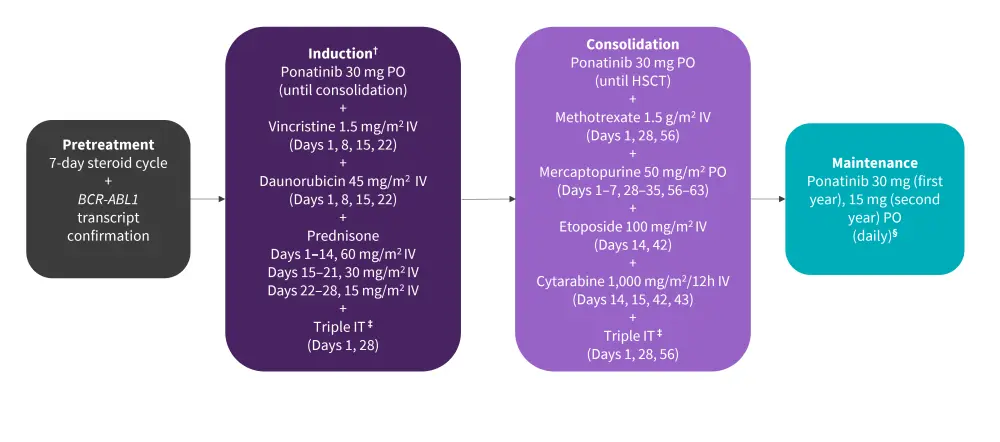
ALL, acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; IT, intrathecal; IV, intravenous; PO, per oral administration.
*Adapted from Ribera, et al.1
†Prephase with prednisone 60 mg/m2 and triple IT was given for a maximum of 1 week, while ALL was fully characterized.
‡Triple IT with methotrexate (15 mg), cytarabine (30 mg), and hydrocortisone (20 mg).
§After alloHSCT, only if molecular disease persisted or reappeared.
Objectives
- Coprimary endpoints were achievement of response (complete hematologic response [CHR], major molecular response [MMR], and complete molecular response [CMR]) post-induction and prior to HSCT, and event-free survival (EFS).
- Secondary endpoints included the rate of patients receiving HSCT in the first CMR, transplant-related mortality (TRM), overall survival (OS), type and frequency of adverse events (AE), and severe AE and the propensity-matched analysis comparing outcomes with those of the ALLPh08 trial (NCT01491763).
Results1
Efficacy
- All patients completed the induction phase and were evaluable for patient response (Figure 2)
- CHR was achieved in 100% of patients
- CMR was achieved in 47% of patients at end of induction, MMR in 17% of patients, and no molecular response in 36% of patients
- Overall, 28 patients progressed to the consolidation portion of the trial, at the end of which 71% were in CMR, 25% were in MMR, and 4% did not achieve a molecular response
- Two patients withdrew from the trial at end-of-consolidation
- AlloHSCT was performed in 26 patients and no patient received autologous HSCT, with the median time from the start of treatment to the transplant was 5.7 months
- Five patients demonstrated molecular relapse, with a median time to onset of 8 months
- Overall, 20/26 patients in continuous CMR did not receive any TKI post-HSCT, with 18 patients remaining in the trial
- 3-year EFS probability was calculated at 70% (95% confidence interval [CI], 51–89%)
- At data cutoff, 29 patients were alive, with the 3-year OS probability being calculated at 96% (95% CI, 89–100%)
The following variables were selected for the propensity score analysis in patients from the PONALFIL and the ALLPh08 trials: age, ECOG Performance Status, white blood cell count, central nervous system involvement at diagnosis, cytogenetics, and BCR-ABL1 isoform. The 3-year OS rates for PONALFIL and ALLPh08 were 96% and 53%, respectively (p = 0.002).
Figure 2. Patient disposition*
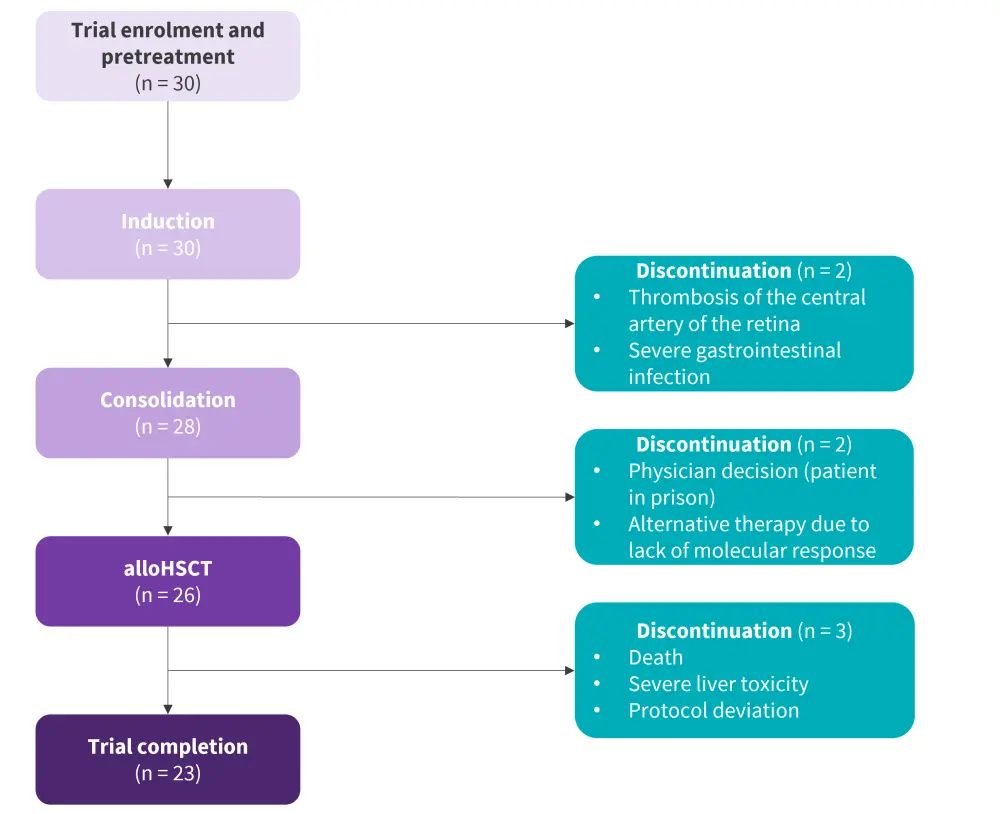
AlloHSCT, allogenic hematopoietic stem cell transplantation.
*Adapted from Ribera, et al.1
Safety
AEs were reported in 20 patients (67%), with 16 patients (53%) experiencing a ≥Grade 3 AE. Three patients discontinued the study due to severe AEs (thrombosis of the central retina artery, severe bowel infection and perforation with peritonitis, and Grade 4 hepatic toxicity after alloHSCT). Transient Grade 2 liver toxicity was reported by 25% of patients who received ponatinib after HSCT.
The most common AEs of any grade were hematologic (28%), gastrointestinal (14%), hepatic (11%), and infection (8%). The most commonly reported ≥Grade 3 AEs were hematologic (42%), hepatic (22%), infection (17%) with only one vascular occlusive event. One patient died of graft-versus-host disease and two patients discontinued the trial each due to severe liver toxicity and protocol deviation.
Conclusion
The results demonstrate a favorable efficacy, alloHSCT performance, encouraging 3-year EFS and OS probabilities and an acceptable safety profile of the combination of ponatinib and standard chemotherapy followed by alloHSCT in adult patients with newly diagnosed Ph+ ALL. Furthermore, propensity score-matched analysis of the PONALFIL and ALLPh08 trials demonstrated a favorable OS rate (96% vs 53%) for ponatinib over imatinib. Taken together, these results showed that the combination of ponatinib with standard induction and consolidation chemotherapy followed by alloHSCT may provide a promising treatment option for adult patients with newly diagnosed Ph+ ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


