All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
ESMO clinical practice guidelines: Targeted therapies for adult acute lymphoblastic leukemia
Do you know... Which of the following treatments is the recommended standard of care for adult patients with newly diagnosed Burkitt lymphoma/leukemia or CD20 plus B-ALL with CD20 expression greater than or equal to 20%?
The use of multi-agent chemotherapy has yielded long-term survival rates of ≥90% in children, 70% in adolescents and young adults, and <20% in elderly patients with acute lymphoblastic leukemia (ALL). This approach is associated with myelotoxicities that can lead to infection and death in the induction and consolidation phases for children (1–3%), adults (≤10%), and elderly patients (≥20%).1
The introduction of immunotherapies such as monoclonal antibodies, targeting cluster of differentiation (CD)20, CD22, or CD19 antigens, have achieved promising response and survival rates in B-cell ALL (B-ALL), including as a monotherapy or combined with chemotherapy for first-line treatment in relapsed/refractory (R/R) disease and patients with minimal residual disease (MRD). Although these treatments are associated with toxicities, they are manageable.
Here, we summarize the European Society for Medical Oncology clinical practice guidelines published by Hoelzer et al.1 in Annals of Oncology, highlighting the recent developments and key recommendations for the use of targeted therapies for the management of newly diagnosed and R/R ALL.
Treatments for newly diagnosed and R/R ALL
The proposed algorithms for the treatment of adults with newly diagnosed or R/R ALL are presented in Figure 1 and Figure 2, respectively.
Figure 1. Treatment algorithm for newly diagnosed ALL*
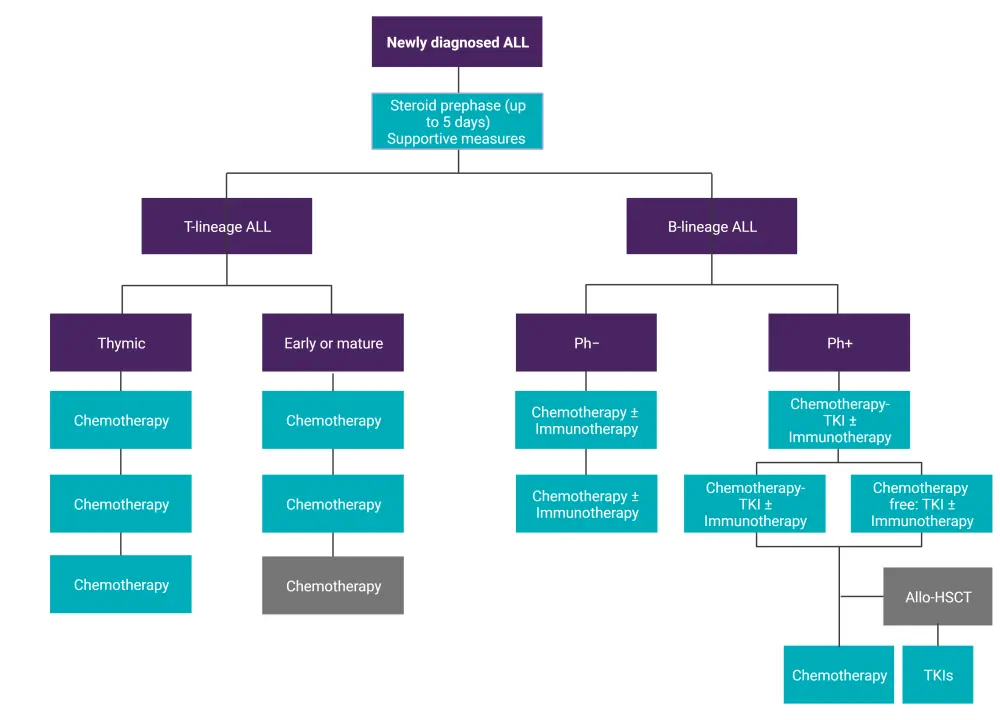
ALL, acute lymphoblastic leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; Ph−, Philadelphia chromosome-negative; Ph+, Philadelphia chromosome-positive; TKI, tyrosine kinase inhibitor.
*Data from Hoelzer, et al.1 Created with BioRender.com.
Figure 2. Treatment algorithm for R/R ALL*
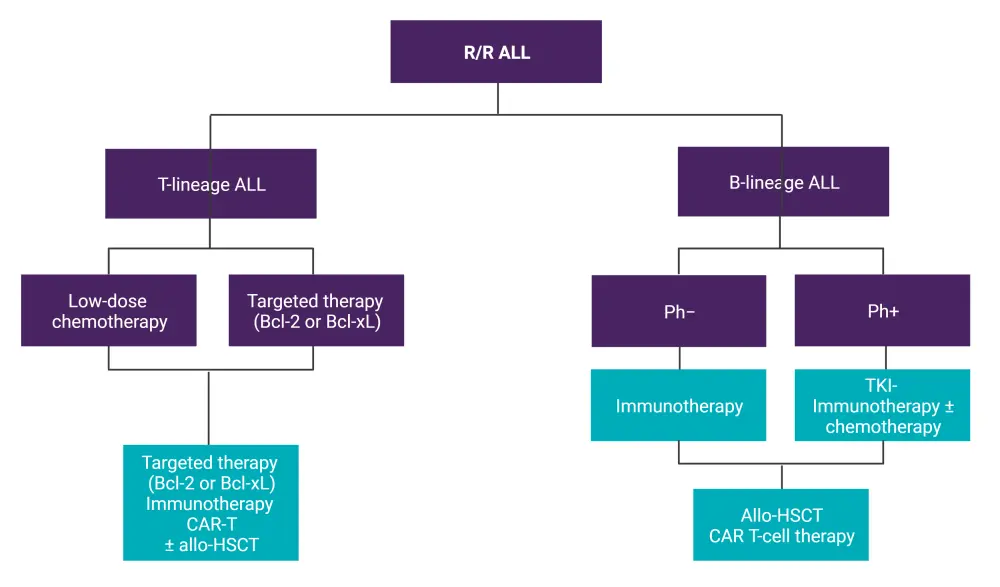
ALL, acute lymphoblastic leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; BCL-2, B-cell lymphoma 2; CAR-T, chimeric antigen receptor T-cell therapy; Ph−, Philadelphia chromosome-negative; Ph+, Philadelphia chromosome-positive; R/R, relapsed/refractory; TKI, tyrosine kinase inhibitor.
*Data from Hoelzer, et al.1 Created with BioRender.com.
Rituximab
Rituximab, an anti-CD20 monoclonal antibody, combined with intensive chemotherapy has demonstrated promising results as a first-line treatment in adults with Burkitt lymphoma/leukemia and newly diagnosed CD20+ B-ALL (Figure 3). Although not yet approved by the European Medicines Agency and U.S. Food and Drug Administration (FDA), the addition of rituximab at a standard dose of 375 mg/m2 intravenously 1 day before chemotherapy is the recommended standard for newly diagnosed adults with Burkitt lymphoma/leukemia or CD20+ B-ALL with CD20 expression ≥20%.
Figure 3. Clinical trials on first-line rituximab plus chemotherapy in adult Burkitt lymphoma/leukemia and CD20+ B-ALL*
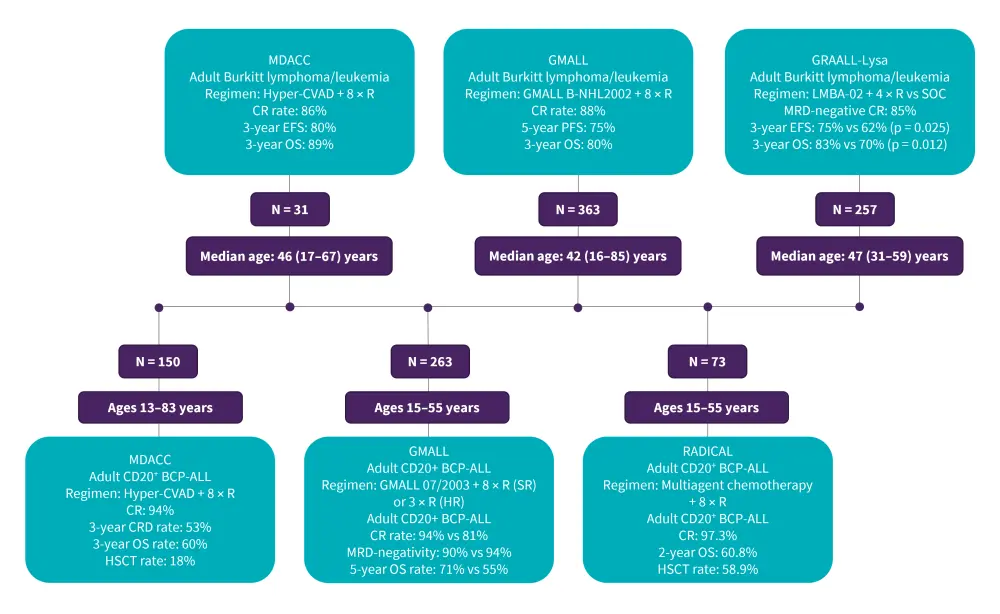
B-ALL, B-cell acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; CR, complete remission; EFS, event-free survival; GMALL, German Multicenter Study Group for Adult Acute Lymphoblastic leukemia; GRAALL, Group for Research on Adult Acute Lymphoblastic Leukemia; Hyper-CVAD, hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MDACC, MD Anderson Cancer Center; MRD, minimal residual disease; R, rituximab; OS, overall survival; PFS, progression-free survival; SOC, standard of care.
*Data from Hoelzer, et al.1
Ofatumumab
Ofatumumab, a second-generation anti-CD20 antibody, plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone has achieved complete remission and MRD-negativity in 98 and 93% of adult patients with CD20+ B-ALL, with a 4-year overall survival (OS) of 68%; this treatment has not proven more effective than hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone plus rituximab in patients with CD20 expression in ≥20% of blast cells, but has demonstrated an improved OS for patients with low CD20 expression (≥1%).
Blinatumomab
Blinatumomab is a CD3/CD19 bispecific T-cell engager antibody that is often associated with cytokine release syndrome and neurotoxicities. It is usually administered at a dose of 9 µg/day in the first week of induction, followed by 28 µg/day subsequently in 28-day continuous infusions.
Blinatumomab plus chemotherapy has improved outcomes for patients with newly diagnosed Philadelphia chromosome-negative B-ALL. For Philadelphia chromosome-positive (Ph+) ALL, dasatinib or ponatinib combined with blinatumomab has obtained high rates of molecular responses and promising OS and event-free survival (EFS) for these patients. Several trials have evaluated blinatumomab in the previously mentioned settings, as well as in R/R disease (Figure 4). Ongoing issues in the management of Ph+ ALL include the investigation of immunotherapies in the early phases of treatment, the identification of patients who can be cured without allogeneic stem cell transplantation, and the role of targeted therapies other than the use of tyrosine kinase inhibitors (TKIs).
Based on the encouraging results from the BLAST (NCT01207388) and AIEOP-BFM (Associazione Italiana di Ematologia Oncologia Pediatrica-Berlin-Frankfurt-Munich) study (NCT03643276), blinatumomab monotherapy is now FDA approved for both pediatric and adult patients with B-ALL in morphological remission with MRD as well as children in first relapse with B-ALL, respectively.
Figure 4. Clinical trials on blinatumomab in newly diagnosed, MRD-positive B-ALL and R/R disease*
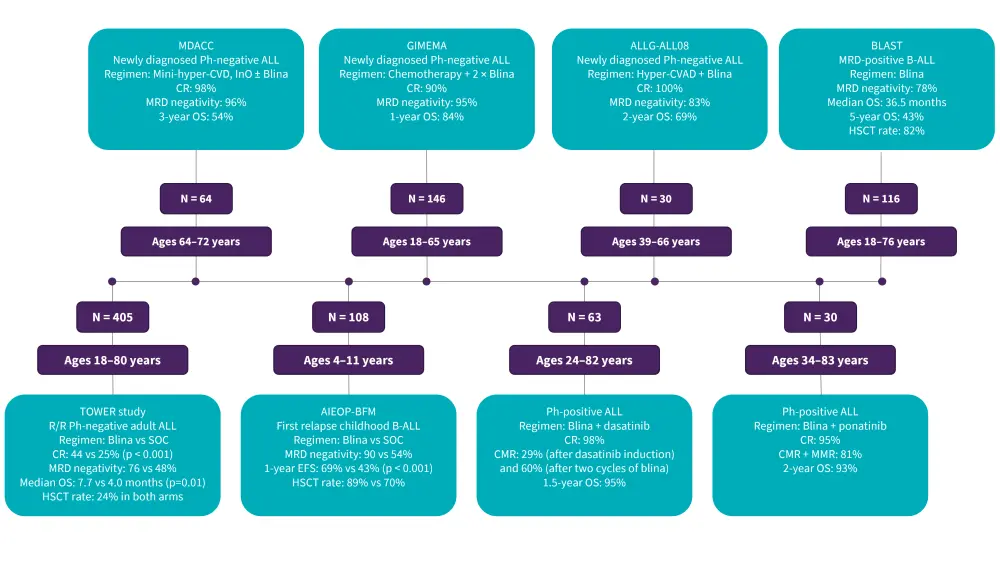
AIEOP-BFM, Associazione Italiana di Ematologia Oncologia Pediatrica-Berlin-Frankfurt-Munich; B-ALL, B-cell acute lymphoblastic leukemia; Blina, blinatumomab; CMR, complete molecular response; CR, complete remission; EFS, event-free survival; GMALL, German Multicenter Study Group for Adult Acute Lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; Hyper-CVAD, hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone; InO, inotuzumab ozogamicin; MDACC, MD Anderson Cancer Center; MMR, major molecular response; MRD, minimal residual disease; OS, overall survival; R/R, relapsed/refractory; SOC, standard of care.
*Data from Hoelzer, et al.1
Inotuzumab ozogamicin
Inotuzumab ozogamicin, an anti-CD22 antibody-drug conjugate, combined with low-intensity chemotherapy has shown encouraging efficacy as a first-line treatment for elderly patients with CD22+ ALL, as well as in adult patients with R/R disease (Figure 5). Given the associated toxicities, inotuzumab ozogamicin (InO) is recommended in patients with R/R B-ALL and no prior liver disease such as a history of portal hypertension, cirrhosis, or chronic liver disease. Based on the results from the phase III INO-VATE study, InO was FDA approved for the treatment of adults with R/R CD22+ B-ALL.
Figure 5. Clinical trials on InO in newly diagnosed, MRD-positive, and R/R disease*
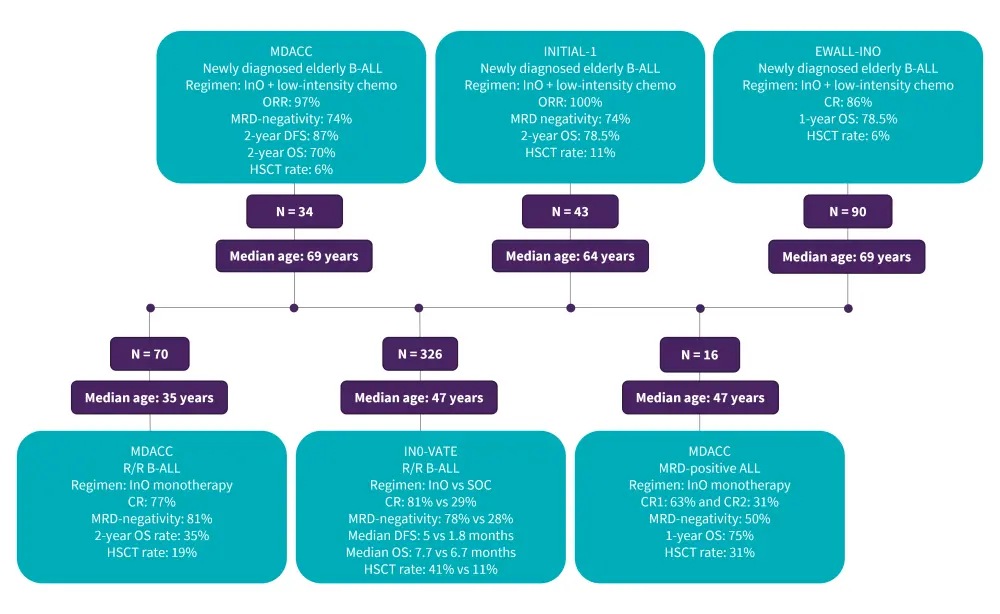
B-ALL, B-cell acute lymphoblastic leukemia; Blina, blinatumomab; CR, complete remission; DFS, disease-free survival; GMALL, German Multicenter Study Group for Adult Acute Lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; Hyper-CVAD, hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone; InO, inotuzumab ozogamicin; MDACC, MD Anderson Cancer Center; MRD, minimal residual disease; ORR, overall response rate; OS, overall survival; R/R, relapsed/refractory.
*Data from Hoelzer, et al.1
CAR T-cell therapy
CD19-directed chimeric antigen receptor (CAR) T-cell therapies have demonstrated excellent response rates of up to 80% in R/R B-ALL regardless of product, manufacture platform, or patient age; this has led to the FDA and the European Medicines Agency approvals of tisagenlecleucel for patients aged ≤25 years and brexucabtagene autoleucel for adults. CAR T-cell therapy is associated with significant immunotoxicities such as cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, the severity of which is based on the CAR T-cell product, and pretreatment disease burden.
There are several CAR-T products currently being investigated in patients with R/R T-ALL, including CD5 CAR-T (Phase I, NCT03081910), NS7CAR (Phase I, NCT04572308), and CD7 CAR-T (Phase I, NCT03690011). The 2-year analysis of donor-derived CD7-directed CAR T-cell therapy in patients with R/R T-cell ALL demonstrated durable efficacy and a 2-year OS rate of 42.3%.
Prior treatment with B-cell targeted immunotherapies can negatively impact CAR-T efficacy. One study by Dourthe et al.,2 reported a shorter EFS and OS from CD19-negative relapse for pediatric and young adults treated with tisagenlecleucel who had prior exposure to blinatumomab. A high disease burden before CAR T-cell therapy is associated with a lower EFS. In the study by Hay et al.,3 patients with elevated lactate dehydrogenase and low platelet count (<100,000/Ul) had a lower EFS, and a recent study showed that ≥5% blasts in the bone marrow at baseline and a high disease burden are associated with significantly lower progression-free survival and higher risk of CD19-negative relapse, respectively.
The omission of fludarabine during CAR-T infusion is associated with lower B-ALL outcomes and a higher risk of cell-mediated CAR T-cell rejection. CAR-T persistence is associated with a prolonged EFS, approaches to increase long-term persistence include the use of 41BBz co-stimulatory endodomains, infusion of CAR-T products enriched for central memory and stem cell memory T-cell populations, and the use of low-affinity CD19 binders. Moreover, CD19 escape is more common in patients with high disease burden; strategies to prevent this include the use of dual-targeted CAR T-cell therapy.
Immune checkpoint inhibitors
Immune checkpoint inhibitors such as CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), PD-1 (programmed cell death protein 1), proteasome, and BCL-2 (B-cell lymphoma 2) inhibitors are new therapies that have been explored in ALL. Pembrolizumab monotherapy has demonstrated limited efficacy in ALL; however, combined with blinatumomab it has yielded promising CR rates of 50% in R/R ALL. Blinatumomab–nivolumab ± ipilimumab has demonstrated high CR rates of 80% in poor-risk R/R CD19+ B-ALL. The BCL2 inhibitor venetoclax plus navitoclax and chemotherapy has demonstrated a CR rate of 60% and a 1-year OS rate of 36% in patients with R/R ALL or lymphoblastic lymphoma, as well as a CR rate of 52% in T-ALL. There are several trials currently investigating venetoclax combined with immunotherapies or ponatinib.
The role of HSCT
Allogeneic hematopoietic stem cell transplantation is recommended for adults with R/R disease, MRD-positive disease, or high-risk groups, usually following bridging therapy with an immunotherapy agent to achieve MRD-negativity. Recent studies have shown that combining immunotherapies such as blinatumomab with TKIs in Ph+ ALL could reduce the need for allogeneic hematopoietic stem cell transplantation.
Conclusion
The updated European Society for Medical Oncology clinical guidelines highlight the latest developments and key recommendations for the use of targeted therapies in the management of newly diagnosed and R/R ALL. Rituximab plus intensive chemotherapy remains the standard for newly diagnosed adults with Burkitt leukemia and CD20+ B-ALL. Consolidation with blinatumomab and blinatumomab plus TKIs remain efficacious agents for Ph-negative and Ph-positive ALL, respectively, and blinatumomab monotherapy is approved for MRD-positive B-ALL and the first relapse of childhood B-ALL.
InO combined with low-intensity chemotherapy are promising regimens for elderly patients with newly diagnosed B-ALL, and InO monotherapy is recommended in patients with R/R ALL with no prior liver disease. CAR T-cell therapies have shown remarkable success in R/R settings, and hematopoietic stem cell transplantation is a recommended curative option for R/R or MRD-positive disease, or high-risk patients following bridging with an immunotherapy agent. Immune checkpoint inhibitors are a recent area of targeted therapies currently being explored that could be promising when combined with immunotherapies.
Despite the advancements in targeted therapies, there is still a need to understand the optimal combination and sequencing of the available targeted therapies, and for more effective therapies for specific subtypes of ALL, such as early T-cell ALL and Ph-like ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

