All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Donor‑derived CD7 CAR T‑cell therapy in R/R T‑ALL: Results from a phase I study
T-lineage acute lymphoblastic leukemia (T-ALL) is an aggressive subtype of ALL which continues to be a difficult-to-treat condition in both pediatric and adult patients.1 Although patient outcomes have significantly improved in recent years, relapsed/refractory (R/R) disease cases continue to have a dismal prognosis.1 The development of chimeric antigen receptor (CAR) T-cell therapies in T-ALL has lagged due to concerns of “fratricide”, relating to the shared expression of target antigens between CAR T-cells and T-ALL.1 Despite these obstacles, recent preclinical results have led to early clinical trials being initiated to investigate CAR T-cell therapies for patients with R/R T-ALL. A phase I trial (ChiCTR2000034762) of donor-derived CD7-directed CAR T-cells in patients with R/R T-ALL has shown promising outcomes at a median follow-up of 6.3 months with a manageable safety profile.2
Here, we summarize a recent publication by Tan et al. in Journal of Hematology and Oncology on the long-term follow-up results of this trial.1
Methods1,2
This was a single-center, open-label, single-arm, first-in-human phase I trial of donor-derived CD7 CAR T-cells for the treatment of R/R T-ALL. Eligible patients were aged 0–70 years, diagnosed with CD7+ R/R T-ALL, with an Eastern Cooperative Oncology Group Performance Status of <3, and without uncontrollable infections or organ failure.
Patients without prior history of stem cell transplant (SCT) were administered with CD7 CAR T-cells as a single intravenous infusion with a target dose of 1 × 106 (±30%) cells/kg of body weight; this was obtained from new donors. Patients with prior SCT were infused with CD7 CAR T-cells obtained from former SCT donors. Infusion of a lower dose (5 × 105 (± 30%) cells/kg body weight) was allowed if the CAR T-cell product did not meet the target dose.
Primary endpoints were dose-limiting toxicities within 21 days and the incidence of adverse events (AEs) within 30 days or between Day 30 and the final visit. Secondary endpoints included overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and CAR T-cell pharmacokinetics in peripheral blood and cerebrospinal fluid.
Results1,2
A total of 20 patients with R/R T-ALL were enrolled between July 18, 2020, and December 21, 2020, and all patients received CD7 CAR T-cell infusion (Figure 1).
Figure 1. Patient disposition*
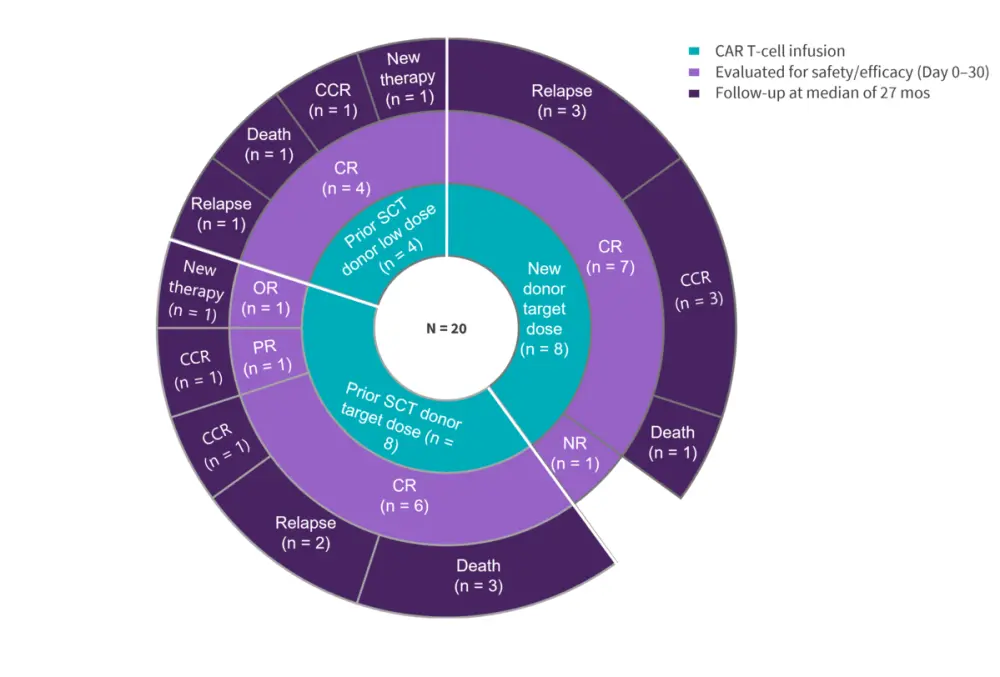
CCR, continuous complete remission; CR, complete remission; D, day; mos, months; NR, no response; PR, partial remission; SCT, stem cell transplantation.
*Data from Tan, et al.1
At the data cut-off of December 20, 2022, the median follow-up time was 27.0 months. The baseline patient characteristics are shown in Table 1.
Table 1. Baseline characteristics*
|
CNSL, central nervous system leukemia; DLI, donor lymphocyte infusion; EMD, extramedullary disease; SCT, stem-cell transplantation. *Adapted from Tan, et al.1 and Pan, et al.2 |
|||
|
Characteristic, % (unless otherwise stated) |
Previous SCT donor |
New donor, |
|
|---|---|---|---|
|
Low dose, |
Target dose, |
||
|
Median age (range), years |
9 (4–18) |
17 (6–33) |
10 (2–43) |
|
Male |
75 |
88 |
63 |
|
Previous therapy |
|||
|
Radiotherapy |
25 |
25 |
13 |
|
Allogeneic SCT |
100 |
100 |
0 |
|
DLI |
100 |
63 |
0 |
|
Primary refractory disease |
0 |
0 |
13 |
|
Bone marrow blasts (by flow cytometry) |
|||
|
>25 |
25 |
13 |
50 |
|
5–25 |
25 |
13 |
38 |
|
0.01–5 |
50 |
50 |
13 |
|
<0.01 |
0 |
25 |
0 |
|
EMDs |
|||
|
CNSL |
0 |
25 |
25 |
|
Diffused |
0 |
25 |
25 |
Safety1
No dose limiting toxicities were observed. The short- and long-term AEs are depicted in Figure 1. Serious AEs reported >30 days post-infusion included five Grade 3 or higher infections and one Grade 4 intestinal graft-versus-host disease (GvHD). Cytopenia occurred in all the 20 treated patients within 30 days, of which 100% were of Grade 3 or higher. Three patients reported late-onset Grade 3 cytopenias at 8, 12.5, and 13 months post-infusion, likely related to the preceding infections.
Figure 2. Adverse events*
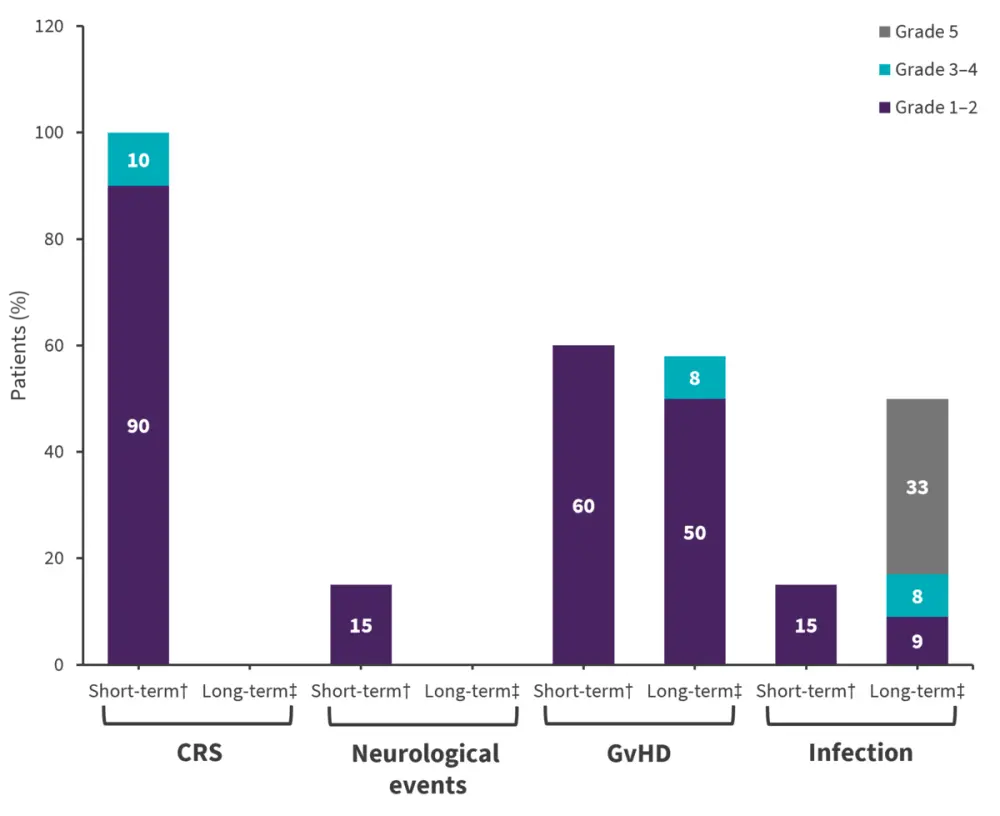
CRS, cytokine release syndrome; GvHD, graft-versus-host disease.
*Data from Tan et al.1
†AEs reported <30 days post-infusion in all 20 treated patients.
‡AEs reported >30 days post-infusion in 12 patients who received prior-SCT donor-derived CAR T-cells but did not further undergo a SCT consolidation.
Of 20 treated patients, five had non-relapse mortality at a median of 6.8 months after treatment. Overall, five deaths were reported, one by engraftment syndrome after SCT consolidation and four by infections in patients without SCT consolidation.
Efficacy1
At Day 30, the treated population ORR was 95% (Figure 2A). One patient with partial remission (PR) at Day 30 achieved CR at Day 45. The median PFS was 11 months and the duration of response was 10.5 months (range, 6.4–12.0 months). The median OS was 18.3 months.
Figure 3. Efficacy outcomes*
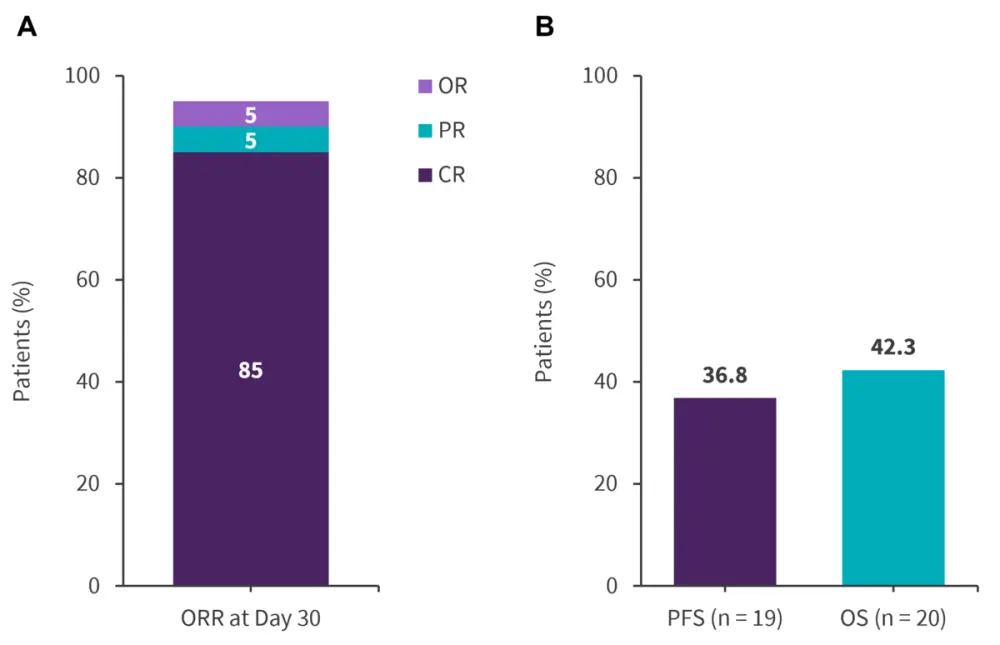
CR, complete remission; OR, objective response; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial remission.
Data from Tan, et al1.
Out of the 19 responders, seven patients receiving new-donor CAR T-cells proceeded to SCT consolidation. A total of six patients (33% of CR patients) relapsed at a median of 6 months post-infusion, of which 4 patients lost CD7 expression on tumor cells.
Pharmacokinetics1
Among 12 patients without SCT consolidation, the median duration of CAR T-cell persistence by flow cytometry was 255 days. CAR T-cells could be detectable in all seven evaluable patients at 6 months post-infusion and two out of four evaluable patients at 12 months post-infusion. Higher peak CAR T-cell count was not associated with the incidence of late-onset severe infection, GvHD, or cytopenias.
T-cell aplasia1
Among 12 patients without SCT consolidation, the number of CD7− T, total T, and natural killer cells progressively increased. By the last visit, total T and natural killer cell counts recovered to normal in seven and six patients at a median time of 1.9 months and 5.1 months, respectively.
Conclusion1
This phase I study of donor-derived anti-CD7 CAR T-cells in patients with R/R T-ALL demonstrated durable efficacy. Disease relapse emerged as a main cause of treatment failure, and severe infection was notably a late-onset AE. A multicenter, open-label, phase II trial (NCT04689659) is ongoing to further investigate the efficacy and safety of donor-derived CD7 CAR T-cells in patients with R/R T-cell leukemia/lymphoma.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


