All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Educational theme | Epidemiology and treatment landscape of Ph-positive ALL
Do you know... Which genetic fusion is responsible for Ph+ ALL?
Introduction
Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is an aggressive form of the disease in which the underlying genetic alterations impact treatment decisions. The incidence of Ph+ ALL increases with age and accounts for 2–5% of cases in children, 20–25% of cases in adults, and more than 50% of cases in patients >50 years of age.1,2
Patients with Ph+ ALL are associated with poor prognosis and standard chemotherapy does not yield adequate responses. With only 66.7% of patients achieving complete response (CR), after which there is a high risk of relapse, the 5-year overall survival (OS) rate is dismal, ranging from 8% to 12%.3
Below, we present a review of the epidemiology and treatment landscape of Ph+ ALL from several recently published articles.
Epidemiology
Ph+ ALL is a high-risk subtype of B-cell precursor ALL caused by a reciprocal rearrangement between chromosome 9 and chromosome 22. Translocation of the ABL1 gene from chromosome 9 and BCR gene from chromosome 22 forms the fusion product BCR-ABL1, an oncogenic gene also known as the Philadelphia chromosome (Figure 1).1 Additional genetic abnormalities, including IKZF1, PAX5, and CDKN2A/2B deletions, have also been identified in Ph+ ALL; frequent alterations in these genes were associated with poor prognosis and outcomes. In an update of the GIMEMA LAL2116 D-ALBA trial (NCT02744768), patients treated with an advanced combination of blinatumomab + dasatinib who had the IKZF1plus genotype (IKZF1 plus PAX5 and/or CDKN2A/2B deletions) showed worse 30-month disease-free survival compared with patients with IKZF1 deletion alone or without IKZF1 deletions (41%, 55%, and 79%, respectively; p = 0.05).2 Patients with Ph+ ALL are at higher risk for central nervous system (CNS) involvement (at least 5%), which remains underdiagnosed and can lead to relapse. The prognosis and outcomes in patients who experience CNS relapse are very poor, with a median OS of 6 months and a projected 5-year OS of zero.4 Prior to the discovery of tyrosine kinase inhibitors (TKIs), the prognosis of Ph+ ALL was poor and 3-year OS was less than 20%; however, with the advent of TKIs and their combination with intensive chemotherapy, prognosis has markedly improved (CR, >90%; associated 5-year OS rate and relapse-free survival rate, 43%).5
Figure 1. Fusion of the ABL1 and BCR genes to form the Philadelphia chromosome*
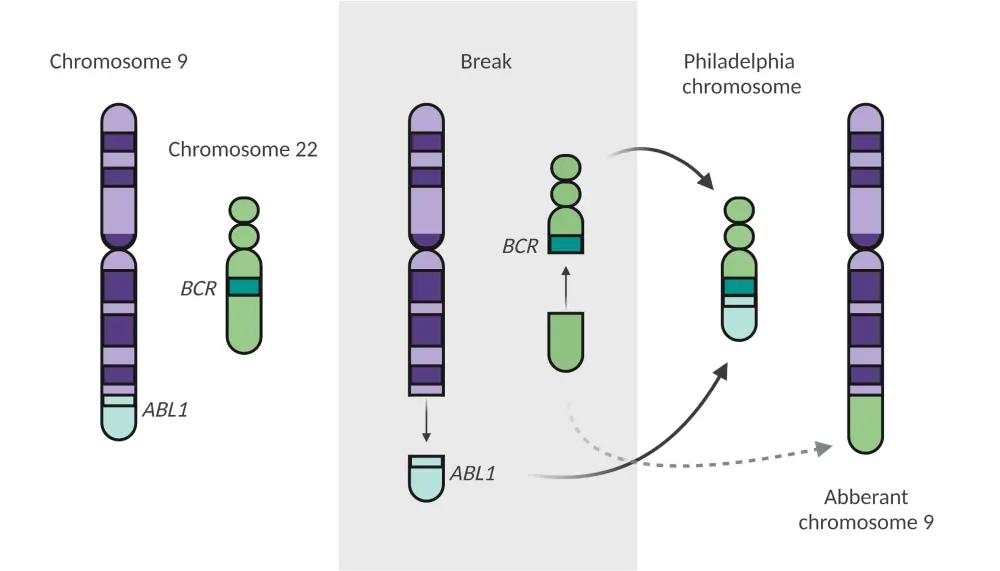
*Adapted from Foà and Chiaretti.1 Created with BioRender.com
Current treatment strategies and future perspectives
For decades, induction chemotherapy followed by allogeneic stem-cell transplant (allo-SCT) was considered the best treatment choice for patients with Ph+ ALL; however, this strategy yielded suboptimal outcomes (estimated long-term survival rates, 10–35%).1 Advances in sequencing technologies have enabled the identification of a diverse range of kinase-activating translocations and mutations that may be amenable to combination therapies in patients with relapsed and refractory (R/R) disease (Figure 2).1 The ALL Hub previously reported on the importance of digital minimal residual disease (MRD) monitoring and treatment outcomes (monotherapy, combination therapies, and chemotherapy-free strategies) in patients with Ph+ ALL. Below, we discuss significant developments in the treatment landscape.
Figure 2. Completed and ongoing trials of treatment for R/R Ph+ ALL*
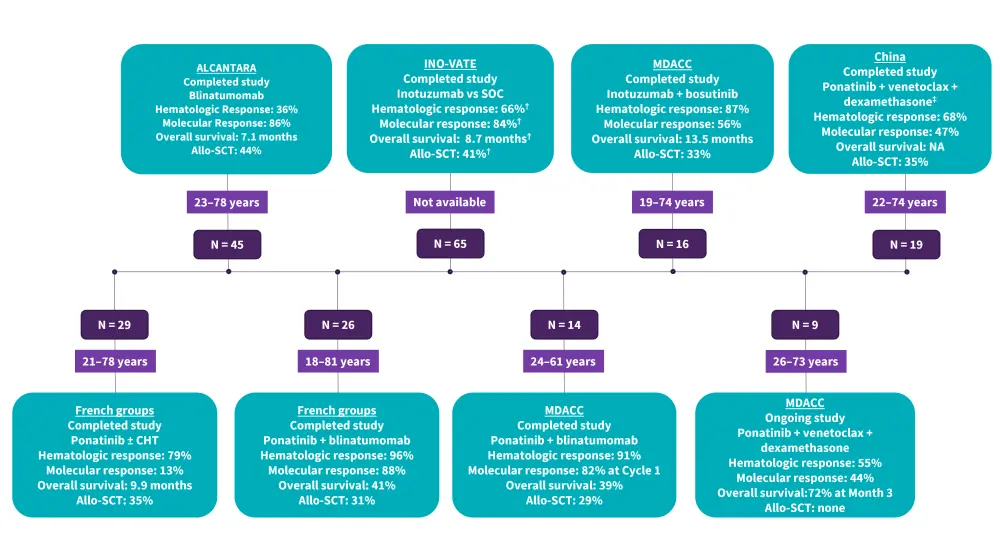
ALL, acute lymphoblastic leukemia; Allo-SCT, allogenic stem cell transplant; CHT, chemotherapy; MDACC, MD Anderson Cancer Center; Ph+, Philadelphia chromosome-positive; R/R, relapsed and refractory; SOC, standard of care.
*Adapted from Foà and Chiaretti.1
†Inotuzumab ozogamicin group.
‡Administered only in patients with T315I mutations.
First-generation TKIs2
Imatinib is a first-generation TKI approved for the treatment of newly diagnosed and R/R Ph+ ALL, clinical trials have revealed that:
- The addition of imatinib to standard chemotherapy has led to significant improvements in complete remission (CR) rates (93%) and OS (30%) in patients with Ph+ ALL.
- Sequential addition of imatinib to intensive chemotherapy during the induction phase resulted in a CR rate of 92% vs 82% in the pre-imatinib cohort, indicating the addition of imatinib in the initial phase of treatment as a viable therapeutic option.
- Addition of imatinib during induction with high-intensity and low-intensity chemotherapy showed similar responses, but high early deaths in the high-intensity chemotherapy arm (6.7% vs 0.7%, p = 0.01).
- Imatinib, when combined with hyper-CVAD, achieved an OS rate of 43% and complete molecular remission (CMR) of 45%. Long-term follow-up studies showed similar OS among patients with or without allo-SCT, suggesting that transplant may not be necessary in all patients with Ph+ ALL.
Second-generation TKIs2
Second-generation TKIs (dasatinib and nilotinib) are more potent at targeting BCR-ABL1 than imatinib; they have shown improved responses and longer survival times in patients with Ph+ ALL, as shown by clinical trials:
- Dasatinib, when combined with hyper-CVAD, achieved a CR and CMR rate of 96% and 65%, respectively, with 17% of patients undergoing allo-SCT in first CR.
- Dasatinib 100 mg/day showed good absorption but poor cerebrospinal fluid penetration; therefore, a higher dose of dasatinib (140 mg/day) was suggested for patients at high risk of CNS relapse.
- Nilotinib, when combined with intensive chemotherapy, achieved an OS and CMR rate of 45% and 83%, respectively, with 53% of patients undergoing allo-SCT in first CR.
- Dasatinib or nilotinib, when combined with vincristine and dexamethasone in older adults (>55 years of age) with Ph+ ALL, showed encouraging CR rates (96% and 94%, respectively) and long-term survival (67 months). These results suggest that long-term survival can be achieved without intensive chemotherapy in older adults who are more susceptible to lower CR rates, higher early mortality, higher relapse rate, and poorer survival compared with younger patients.
- A phase III study in pediatric patients revealed significantly better outcomes of dasatinib compared to imatinib (4-year event-free survival: 71% vs 49%, respectively; p = 0.005; 4-year OS, 88.4% vs 69.2%, respectively; p = 0.04; cumulative risk of relapse, 19.8% and 34.4%, respectively; p = 0.01).
These encouraging results showed that second-generation TKIs, when combined with intensive or low-intensity chemotherapy, can result in higher CMR rates compared with imatinib-based regimens.
Overall, the data support the use of second-generation TKI vs first-generation for better outcomes in patients with Ph+ ALL; however, patients treated with either dasatinib or nilotinib relapsed due to the presence of T315I resistance mutation.
Third-generation TKIs2
Ponatinib, a third-generation TKI, can effectively target Ph+ ALL harboring the T315I mutation and is an option for second-line treatment; however, toxicity (arterial occlusive events and pancreatitis) remains a concern. Clinical trials have revealed that:
- Ponatinib, when combined with hyper-CVAD, achieved a CR rate of 100% (CMR 84%) and 22% of the patients underwent allo-SCT in first CR.
- A recent propensity-score matching phase II study, in newly diagnosed patients with Ph+ ALL, compared hyper-CVAD + dasatinib vs hyper-CVAD + ponatinib and revealed significantly improved outcomes in the ponatinib cohort compared to the dasatinib cohort (3-month CMR, 84% vs 63%, respectively; 3-month OS, 83% vs 56%, respectively; p = 0.03).
- Ponatinib + induction chemotherapy (vincristine, daunorubicin, and prednisone), followed by consolidation (high-dose methotrexate, cytarabine, mercaptopurine, and etoposide) showed promising results in patients aged 19–50 years with the post-induction CR and CMR rates of 100% and 47%, respectively.
- Ponatinib combined with steroids resulted in a high response rate (CMR, 41%) in older patients (≥60 years) who would otherwise be at high risk or ineligible for intensive chemotherapy. Treatment-emergent adverse events included cardiac (29.5%) and vascular (27.3%) events which can be managed by using a lower dose of ponatinib and the preventive use of aspirin and statins.
Combination with TKIs
Blinatumomab is an anti-CD3/CD19 bispecific antibody approved by the U.S. Food and Drug Administration (U.S. FDA) in 2017 as monotherapy for the treatment of CD19-positive R/R ALL. Clinical trials have revealed that:
- Blinatumomab use in patients with R/R Ph+ B-ALL, in whom prior TKI-based therapy did not yield a response, showed favorable outcomes with a CR and CR with partial hematologic recovery (CRi) rate of 36% (CMR, 88%) (Figure 2).1,2
- Combining blinatumomab + TKIs showed favorable outcomes in patients with Ph+ ALL (CMR, 75%; 1-year OS, 73%).2
- Sequential treatment with corticosteroids + dasatinib for 85 days, followed by 2–5 cycles of blinatumomab, resulted in a significant increase in the rate of molecular response from 60% to 29% by the end of dasatinib induction therapy. Despite the existence of T315I and E255K mutations, patients responded well to blinatumomab and 50% of patients underwent allo-SCT in CR. A small number of patients (n = 4) had isolated CNS and hematologic relapses.2
- The chemotherapy-free combination of ponatinib and blinatumomab in the frontline setting showed improved efficacy and safety outcomes (complete response [CR]/Cri, 95%; CMR after 1 cycle and overall, 61% and 86%, respectively); only 1 patient underwent allo-SCT and no deaths and relapses were recorded (Figure 2).
These encouraging results demonstrate the potential of combining ponatinib and blinatumomab as a chemotherapy-free treatment in newly diagnosed patients with Ph+ ALL. This approach can also protect newly diagnosed patients from allo-SCT and the serious toxic effects of chemotherapy.2
Inotuzumab ozogamicin is a humanized anti-CD22 monoclonal antibody, conjugated to calicheamicin, approved for the treatment of R/R ALL. Inotuzumab ozogamicin showed promising activity when combined with a third-generation TKI (bosutinib) in patients with Ph+ ALL.
Initial reports of the combination showed a CR/CRi of 83% in a modest cohort of 18 patients with R/R Ph+ B-ALL. The CMR rate was 53% in the 15 patients evaluated.6
The BCL2 inhibitor venetoclax, when combined with TKIs, showed synergistic in vitro antileukemic activity. A phase II study of the combination of ponatinib, venetoclax, and dexamethasone is ongoing in patients with relapsed or refractory Ph+ ALL (NCT03576547).1
Key challenges and possible solutions1
Despite the development of advanced treatment approaches, a proportion of patients still relapse after allo-SCT as well as treatment with a combination of chemotherapy and TKIs. Persistent or recurrent MRD, late and isolated CNS relapse and presence of IKZF1plus genotype are the main reasons for relapse. Further challenges and possible approaches to overcome these are outlined below (Table 1).
Table 1. Key challenges and possible solutions in the management of Ph+ ALL*
|
ALL, acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CNS, central nervous system; ITC, intrathecal chemotherapy; MRD, minimum residual disease; PCR, polymerase chain reaction; Ph+, Philadelphia chromosome-positive; TKI, tyrosine kinase inhibitor. |
|
|
Challenges |
Possible solutions |
|---|---|
|
Delay in Ph+ chromosome detection leads to use of inappropriate treatment strategies |
Testing to be performed immediately after diagnosis of ALL |
|
Unidentified IKZF1, CDKN2A/2B, and/or PAX5 deletions |
To use more sensitive methods, such as real-time quantitative PCR assay, to identify specific aberrations |
|
Treatment requires out-of-pocket payments, limiting evaluation for patients in many countries |
Early treatment initiation with oral TKI can reduce the cost of treatment in the long term |
|
Lack of appropriate and timely MRD assessment results in recurrence and relapse of the disease |
More precise methods such as droplet digital PCR should be used for accurate measurement of MRD and MRD should be monitored throughout treatment |
|
Disease features such as hyperleukocytosis increase the risk of CNS relapse |
Trial data shows that the addition of TKIs to the backbone of ALL therapies, including intrathecal chemotherapy, can significantly improve survival and reduce CNS relapse |
|
Limited global availability of advanced treatment options (blinatumomab, Inotuzumab ozogamicin, and CAR T-cells) |
— |
|
Delay between sample collection and results |
— |
Conclusion
Ph+ ALL is emerging as a prevalent subtype of B-ALL. It is defined by the BCR-ABL1 fusion gene and associated with a poor prognosis, with high rates of conventional treatment failure and relapse. Third-generation TKIs and chemotherapy-free combinations, including TKIs + blinatumomab/inotuzumab ozogamicin are promising for newly diagnosed patients with Ph+ ALL who would otherwise be at high risk for intensive chemotherapy. Additionally, patients should be closely monitored for MRD and isolated CNS disease to identify an early disease recurrence and prevent a relapse. Recent advancements demonstrate the opportunity to consider targeted therapeutic approaches that aim to reduce relapse rates and improve long-term survival for patients with Ph+ ALL of all ages.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

