All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Do you know... Which of the following is an approved drug for the treatment of patients with T-ALL?
As part of our ongoing education theme series on T-cell acute lymphoblastic leukemia (T-ALL), the ALL Hub is happy to present an overview of the T-ALL treatment landscape. Here, we focus on established therapies, current challenges, and future perspectives. Previous editions in this series have addressed the genetic heterogeneity, incidence, prognosis, and current diagnostic approaches and risk stratification of T-ALL.
The treatment landscape for T-ALL is advancing; however, this rare and aggressive subtype of ALL remains a challenging disease for both adult and pediatric patients. Although patient outcomes have vastly improved in recent years, cases of relapsed/refractory (R/R) disease remain a particular challenge to treat, with those who cannot tolerate intensive treatment protocols facing dismal outcomes.1
Currently, there is no consensus approach for the treatment of T-ALL. Clinical treatment regimens for T-ALL are largely similar to those used in the treatment of B-cell ALL and those used for pediatric T-ALL patients are comparable to those selected for adult patients. Commonly used broad-spectrum chemotherapeutic drugs are associated with poor efficacy and a range of adverse effects, with there being few targeted therapies available for T-ALL.2
As addressed in previous editions of this educational theme series, aberrant signaling pathways (NOTCH, PI3K/AKT/mTOR, NF-kB, and Wnt) are a hallmark of T-ALL etiology; therefore, small molecule inhibitors targeting the major kinase components of these signaling pathways have received much attention in recent years.2
The development of novel efficacious treatments is a significant unmet need for this patient population. Potential future treatment options for R/R T-ALL are summarized in Figure 1.
Figure 1. Potential treatments for R/R T-ALL*
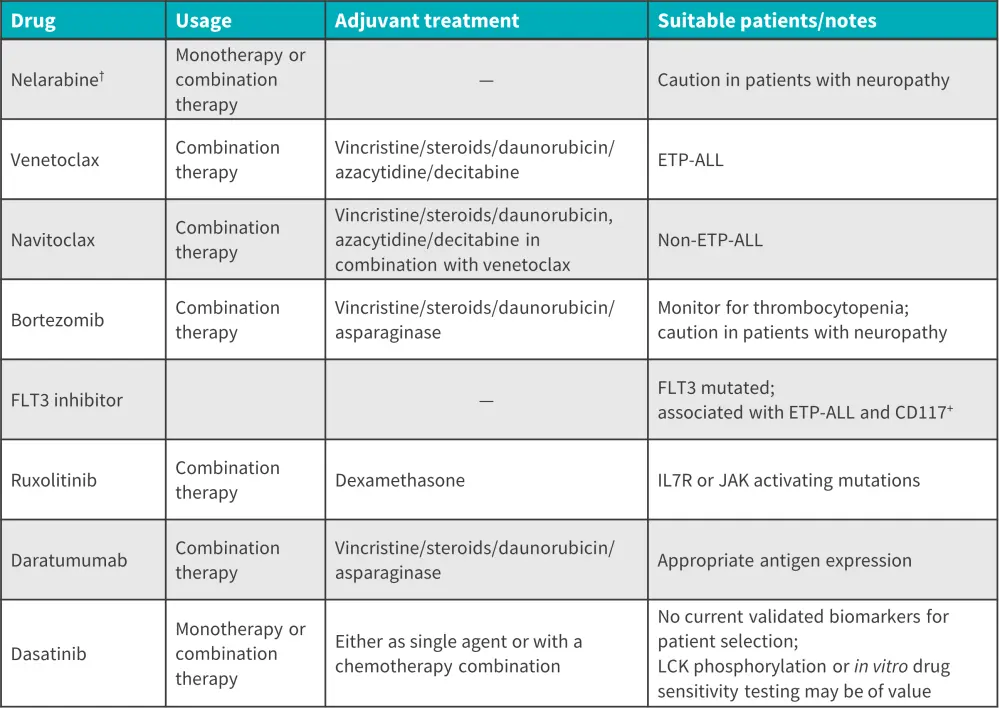
ALL, acute lymphoblastic leukemia; CD, cluster of differentiation; ETP, early T-cell precursor; JAK, Janus kinase; LCK, lymphocyte-specific protein tyrosine kinase.
*Adapted from Pocock, et al.1
†FDA approved
Chemotherapy
Most drugs used for the treatment of adult T-ALL are broad-spectrum chemotherapeutic agents and, although these agents have demonstrated promising recovery rates in pediatric patients, the results in adult patients have been sub-optimal. The continued use of these broad-spectrum agents can lead to an increase in drug resistance, along with a range of adverse drug reactions.2
Younger adult patients have been shown to benefit from a pediatric-inspired intensive regimen, whilst older and unfit patients often receive appropriate multiagent chemotherapy. A number of studies have shown that adolescents and young adults (AYAs) treated with pediatric chemotherapy regimens have better outcomes than those treated with adult-intensive chemotherapy regimens.3
For older patients, the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine) can provide clinical benefit. While intensive, this combination has proven to be well tolerated in fit older patients.
Nelarabine, a soluble prodrug of guanine arabinoside, is the only approved drug for the treatment of T-ALL and has shown clinically meaningful responses in a substantial minority of patients with R/R T-ALL. 3 In the frontline setting, nelarabine has been used in combination with chemotherapy in both pediatric and adult patients. In the pediatric setting, it has demonstrated promising efficacy as a single agent, with neurotoxicity being the most common dose-limiting toxicity. In adult patients, an alternate-day dosing schedule was shown to limit neurotoxicity while maintaining an acceptable response rate. Further data report that nelarabine in combination with etoposide and cyclophosphamide achieved a complete remission rate of 71% in relapsed patients.1 Nelarabine, in combination with hyper-CVAD, has also achieved a CR rate of 94% as an initial therapy in adult patients.2
Historically, standard lymphoma regimens had been used to treat T-ALL, but therapy has now evolved to the use of ALL regimens involving induction, consolidation, delayed intensification, and maintenance phases; additional CNS prophylaxis with high-dose chemotherapy and intrathecal therapy have been used recent years.3 Also, intensive chemotherapy is occasionally supplemented with cranial radiotherapy in pediatric patients.4
Transplant therapy
Hematopoietic cell transplantation (HCT) plays an important role in the treatment of patients with high-risk or R/R T-ALL5; however, even in patients who are responsive to chemotherapeutic agents and have received consolidation therapy, clinical outcomes can remain poor. In older patients with T-ALL, studies have shown that allogenic HCT in combination with reduced-intensity conditioning regimens may provide clinical benefit. Conversely, autologous HCT has been shown to have limited utility in the context of T-ALL.3 Furthermore, HCT depends on the availability of donors and the patient's health.6 Umbilical cord blood HCT is emerging as a potential therapy for patients with T-ALL; however, experience is limited with this donor source.3
Targeted therapy
Targeting signaling pathways involved in the pathogenesis of T-ALL may be a useful strategy in the management of T-ALL. Targeted therapeutic approaches may allow us to overcome some of the challenges of broad-spectrum chemotherapeutic agents.
The potential targeted agents currently under consideration for T-ALL treatment include tyrosine kinase inhibitors, purine analogues, inhibitors of pathogenic signaling pathways (NOTCH-FBXW7 pathway antagonists, IL7-IL7R pathway antagonists, PI3K-Akt-mTOR pathway antagonists, RAS-RAF-MEK-ERK pathway antagonists, and Hedgehog pathway antagonists), and other targeted agents for concomitant use. Advantages of using targeted agents over conventional chemotherapeutic approaches include their higher survival rates, reduced adverse drug reaction rates, and lower toxicity. The potential of targeted drugs heralds a change from a universal to a more individualized treatment approach; with these new methods, it is hoped that T-ALL has a higher chance of being cured.2
CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cells are autologous T-lymphocytes that have undergone genetic modification to express binding sites for particular antibodies. With impressive efficacy, CAR T-cells are a novel and promising immunotherapeutic strategy for treating ALL. Allotransplantation and multi-target CAR T-cell therapy have emerged as the next options for remission therapy in the treatment of clinical ALL. Currently, CAR T-cells are primarily used in the treatment of B-cell ALL, with significant results being reported in the treatment of CD19 and CD22 targets. Experiments have confirmed the overexpression of multiple CD markers, including CD5, CD7, CD19, CD33, CD38, CD52, and CD99, in T-cell malignant tumors. CAR-T cell therapy development in T-cell ALL has lagged behind its development in B-cell ALL because of concerns of “fratricide” related to shared expression of target antigens between CARTs and T-ALL. These concerns are being overcome with novel approaches which are most advanced in the development of CD7-directed CAR-T cells. CAR T-cell therapy is however, also associated with cytokine release syndrome and immune effector cell-related neurotoxicity syndrome which should be considered in treatment planning.2
Future perspectives
Going forward, targeted therapy is expected to play a greater role in treating patients with T-ALL; however, limitations are beginning to emerge, such as therapeutic resistance in signaling pathway-targeted drugs. The use of targeted drugs in combination with biological agents will likely offer a path to significant breakthroughs in T-ALL treatment. In addition, adjusting the dosing schedule, the intensity and duration of inhibition of target inhibitors, and using intermittent dosing schedules could optimize existing treatment regimens.2 The application of novel techniques such as gene sequencing, drug sensitivity testing, and kinase activity analysis may help to further elucidate individual patient needs and provide a truly individualized treatment approach.2 Table 2 provides details of several promising novel therapies that may be approved for the treatment of T-ALL. As CAR T-cell therapy is predicted to become a viable treatment option in the years to come, these molecules could be effective binding sites for future CAR T-cell products.2
Figure 2. Promising future targets and drugs in novel T-ALL therapies*
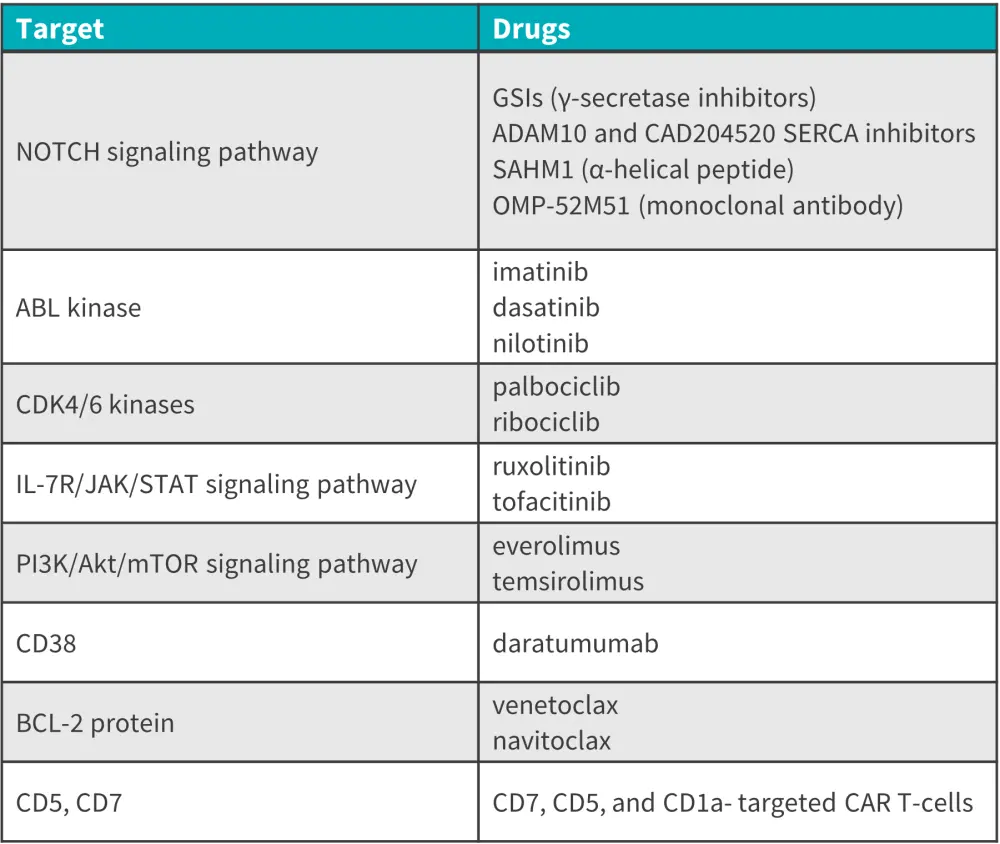
BCL-2, B-cell lymphoma-2; CAR, chimeric antigen receptor; CD, cluster of differentiation; IL, interleukin; JAK, Janus kinase; mTOR, mechanistic target of rapamycin; OMP, olfactory protein marker; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; STAT, signal transducer and activator of transcription.
*Adapted from Lato, et al.4
Conclusion
Currently, nelarabine is the only medication to be approved and specifically indicated for the treatment of T-ALL. Despite advances in recent years, there remains a significant unmet need for the development of novel efficacious treatments for this patient population. Although there is no unanimously adopted treatment approach for T-ALL, a number of drugs and strategies (particularly targeted therapy) are currently under investigation; many may lead to effective clinical therapies, offering hope to patients and healthcare providers.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Mark Litzow
Mark Litzow
