All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Frontline treatments for older patients with Ph− B-ALL
Acute lymphoblastic leukemia (ALL) represents 5% of all newly diagnosed leukemias in older patients aged 55 to 70 years old.1 With recent advances largely centered around the younger population, older patients have seen limited prognostic benefit. Ineligible for unmodified pediatric-based therapies, survival of elderly patients remains poor with age-adapted, dose-reduced chemotherapy regimens, even after moderately intensive doses.1
Below is a summary of recent data from abstracts presented at the 63rd American Society of Hematology (ASH) Annual Meeting and Exhibition regarding novel frontline treatment regimens for older patients with Philadelphia chromosome-negative (Ph−) B-ALL.
Fractionated inotuzumab ozogamicin plus low-intensity chemotherapy for patients with ALL: results from the EWALL-INO study2
Background
Inotuzumab ozogamicin (INO), an anti-CD22 antibody conjugated to calicheamicin, is approved for the treatment of relapsed/refractory (R/R) B-cell precursor (BCP) ALL in adults. However, this treatment is associated with a major adverse event: sinusoidal obstruction syndrome (SOS). A previous study by Kantarjian et al., published in Lancet Oncology, (NCT01371630) established INO plus mini-hyper-CVD chemotherapy as a safe and effective first-line treatment in older patients with newly diagnosed ALL. Due to incidences of SOS, the total doses were fixed at 1.3 mg/m² for Cycle 1 followed by three cycles at 1 mg/m². Patrice Chevallier2 presented data from the EWALL-INO phase II study (NCT03249870), which aimed to assess the activity and safety of fractionated INO at a reduced dosage in combination with low-intensity chemotherapy as frontline therapy for older patients with CD22+ Ph− BCP-ALL.
Study design
EWALL-INO is an ongoing, single-arm, multicenter study conducted in European centers.
Eligibility criteria is as follows: patients with newly diagnosed CD22+ Ph− BCP-ALL without central nervous system involvement; aged ≥55 years; ECOG performance status ≤2; AST and ALT ≤2.5, bilirubin and creatinine ≤1.5.
The study design is shown in Figure 1. POMP maintenance was given for 18 months, and included dexamethasone, vincristine, and methotrexate.
Figure 1. EWALL-INO study design*
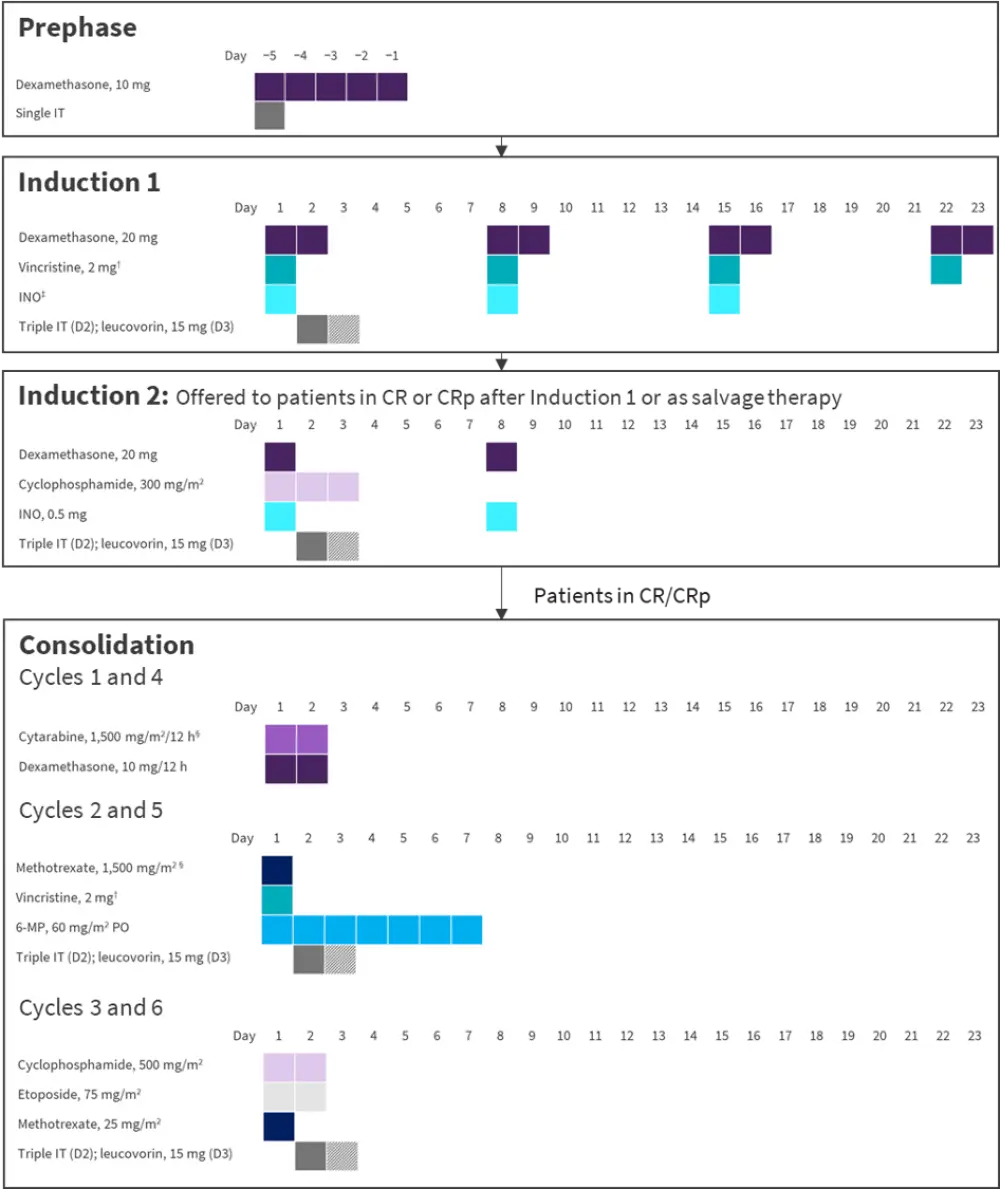
6-MP, 6-mercaptopurine; CR, complete remission; CRp, CR with platelets <100 G/L; D, day; INO, inotuzumab ozogamicin; IT, intrathecal injection.
*Adapted from Chevallier, et al. 2021.2
†Patients aged >70 years received 1 mg.
‡D1, 0.8 mg/m²; D8 and 15, 0.5 mg/m².
§With dose adaption according to age and estimated glomerular filtration rate.
Results
A total of 90 patients were included in the first analysis, which had a minimum follow-up period of 4 months. Median age was 69 years, and median CD22 expression was 86.5%. Genetic subtypes included low hypodiploid/near triploid (28%), Ph-like (11%), KMT2A rearranged (10%), and others (51%).
Efficacy
- The median follow-up was 1.18 years (range, 0.3–3.5).
- The CR rates after Induction 1 and Induction 2 were 86.7% and 88.8%, respectively.
- Of the 67 evaluable patients for MRD <10−4 after Induction 2, 73% were negative.
- The 1-year overall survival, relapse-free survival, and incidence of relapse were 78%, 76%, and 16%, respectively.
- Patients with KMT2A rearrangements had the worst overall survival because of a higher incidence of relapse.
Safety
- Eight patients (8.8%) had Grade 3–4 liver toxicity.
- Three patients (3.3%) had SOS: two during Induction 1 and one after transplant.
- Three patients died during induction, and 29 patients died during follow-up (16 due to relapse and 13 due to adverse events, including one COVID-19 fatal infection).
Conclusion
Low-dosage, fractioned INO combined with low intensity chemotherapy has shown promising efficacy as a frontline therapy for older patients with CD22+ Ph− BCP-ALL, with a CR rate of 88.8% following Induction 2, and a 1-year OS of 78%, as well as a good safety profile, with a low SOS rate of 3.3%. A limitation of this analysis is the small patient population; the investigators aim to enroll 130 patients and explore the impact of prognostic factors, including MRD.
Inotuzumab ozogamicin induction combined with chemotherapy-based consolidation and maintenance therapy in patients aged >56 years with ALL: results from the INITIAL-1 trial1
Background
Matthias Stelljes1 presented the results of the multicenter, phase II INITIAL-1 trial (NCT03460522), which assessed the efficacy and safety profile of three cycles of INO as a frontline induction therapy followed by conventional consolidation for patients aged >56 years with newly diagnosed CD22-positive, Ph/BCR-ABL negative acute B-precursor ALL.
Study design
Three induction cycles were given, and patients who reached a CR were offered five conventional consolidation therapies (methotrexate/asparaginase three times; AraC two times) and one reinduction therapy (idarubicine, AraC, cyclophosphamide, and dexamethasone) in combination with rituximab (for CD20+ ALL), followed by a maintenance therapy with methotrexate (Figure 2).
Figure 2. INITIAL-1 study design*
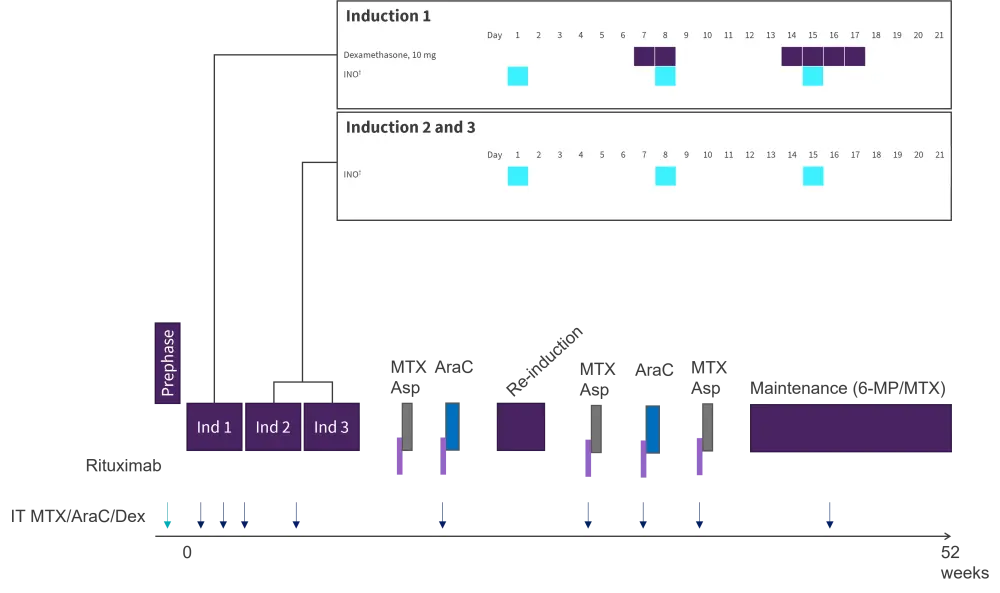
6-MP, 6-mercaptopurine; AraC, cytarabine; Asp, asparaginase; dex, dexamethasone; ind, induction; INO, inotuzumab ozogamicin; IT, intrathecal injection; MTX, methotrexate.
*Adapted from Stelljes, et al. 2021.1
†D1, 0.8 mg/m²; D8 and 15, 0.5 mg/m².
The primary endpoint was event free survival (EFS) at the 12-month follow-up. An event was defined as persisting bone marrow blasts after two cycles of INO, relapse, or death. An event rate of ≤40% at the 12-month follow-up was considered very promising for further evaluation.
Results
Forty-three out of the 45 patients enrolled in the study were evaluable. Median age was 64 years (range, 56–80), and most patients (88%) had common ALL. Median CD22 expression on blast cells was 69%. Response rates after induction therapy are shown in Table 1.
Table 1. Treatment response*
|
CR, complete remission; CRi, complete remission with incomplete count recovery; HSCT, hematopoietic stem cell transplantation; INO, inotuzumab ozogamicin; MRD, minimal residual disease. |
|
|
Evaluable for hematologic remission |
N = 43 |
|---|---|
|
CR/ CRi after two induction cycles, % |
100 |
|
Patients receiving three cycles of INO, % |
94 |
|
Early deaths within the first 3 months |
0 |
|
Evaluable for MRD |
|
|
MRD− after 2nd induction cycle, % |
53 |
|
MRD <10-4 after the 2nd induction cycle, % |
35 |
|
MRD− after 3rd induction cycle, % |
71†‡ |
|
MRD+ >10-4 (molecular failure), % |
12† |
|
MRD+ <10-4, % |
17† |
|
Hematologic relapse, n |
3 |
|
Allogeneic HSCT in the first remission, n |
4 |
|
Allogeneic HSCT after relapse, n |
1 |
- A total of seven events have occurred to date. During the first year, three deaths were reported in patients with CR and one patient relapsed with ALL. During the second year, one death occurred in a patient who was in CR and two patients relapsed.
- With a median follow-up of 422 days, the OS at 1 year was 91% (95% confidence interval [CI], 80–100%) and OS at two years was 77% (95% CI, 57–96%).
Safety
The most frequent Grade ≥3 adverse events (AEs) reported during induction were leukocytopenia, anemia, and thrombocytopenia (Table 2). To date, one case of suspected veno-occlusive disease has been reported.
Table 2. Grade ≥3 AEs during induction therapy*
|
AE, adverse event; GOT; glutamic oxalacetic transaminase; GPT; glutamic pyruvic transaminase. †Missing data for two patients. |
|||
|
Grade ≥3 AEs >5% |
Induction 1 |
Induction 2 |
Induction 3 |
|---|---|---|---|
|
Leukocytopenia, % |
60 |
12 |
2 |
|
Anemia, % |
28 |
2 |
0 |
|
Thrombocytopenia, % |
35 |
7 |
2 |
|
Elevation of GOT/GPT, % |
9 |
0 |
0 |
|
Elevation of bilirubin, % |
2 |
0 |
0 |
|
Hyperglycemia, % |
12 |
10 |
2 |
|
Hypophosphatemia, % |
5 |
2 |
2 |
|
Hypokalemia, % |
2 |
2 |
0 |
|
Hyperuricemia, % |
5 |
2 |
0 |
|
Infection, % |
2 |
0 |
0 |
|
Fatigue, % |
0 |
0 |
2 |
Conclusion
The results from this study show promise, with a high CR/CRi after only two INO induction cycles and high MRD negativity rates after the second and third induction therapy. Further investigation of this novel induction regimen is warranted.
Dose-reduced chemotherapy plus blinatumomab for newly diagnosed older patients with ALL: results of the ongoing GMALL BOLD trial.
The latest results from the German Multicenter Adult Acute Lymphoblastic Leukemia (GMALL) BOLD trial (NCT 03480438) were presented by Nicola Gökbuget.3 This study also aims to address the unmet need of older patients with newly diagnosed ALL who are ineligible for unmodified pediatric-based therapies. It was previously demonstrated in the GMALL elderly protocol (n = 412) that patients receiving dose-reduced chemotherapy could reach a CR rate of 75%, with 16% early death, but the 3-year OS was only 30%. Therefore, this trial aims to evaluate the CD19-directed, bi-specific T-cell engager, blinatumomab, in sequence with chemotherapy in this patient population. The study was designed for blinatumomab to replace several cycles of chemotherapy as part of the standard GMALL protocol for older patients.
Study design
The ongoing study has recruited 34 patients from 13 German centers to date. Patients aged 56–76 years with CD19-positive, Ph− B-precursor ALL were eligible. The primary endpoint was CR after induction (one dose of reduced cycle chemotherapy (IP1) and one cycle of blinatumomab). The key secondary endpoint was a molecular response with a goal to reduce induction mortality and relapse rate. Figure 3 shows the study design. The median follow-up was 363 days (range, 26–1,001).
Figure 3. GMALL BOLD study design*
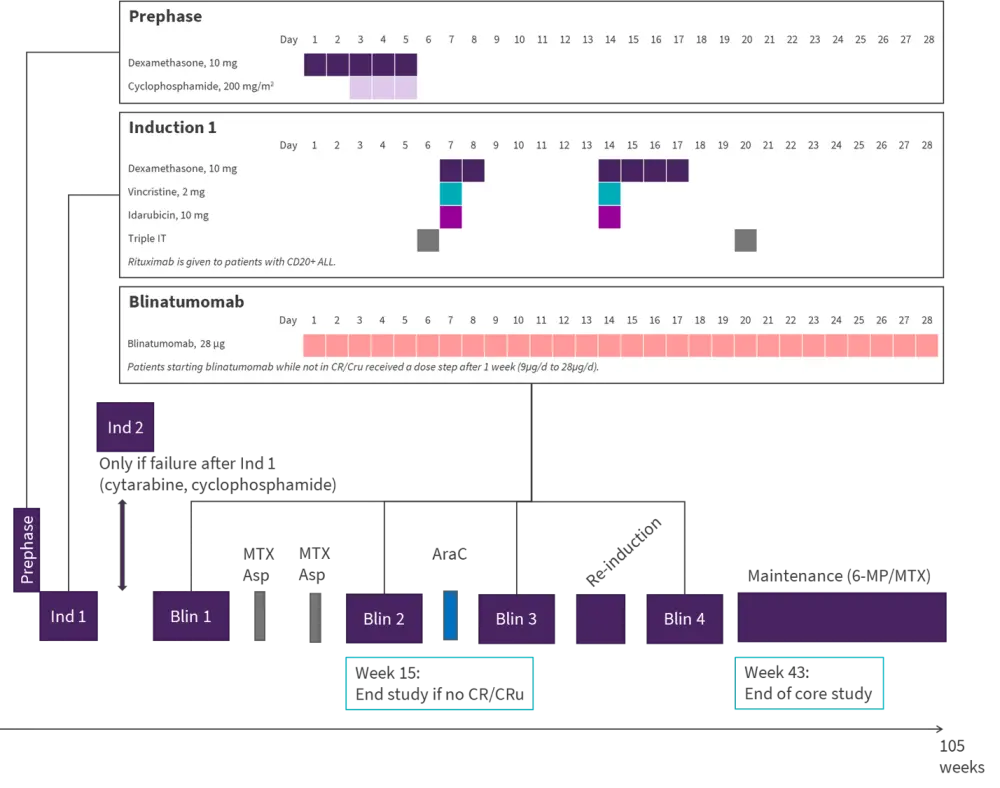
6-MP, 6-mercaptopurin; AraC, cytarabine; Asp, asparaginase; CR/CRu, complete remission/unconfirmed; ind, induction; IT, intrathecal injection; MTX, methotrexate.
*Adapted from Gökbuget, et al. 2021.2
Results
The median age was 65 years. Common/pre-B-ALL and pro-B-ALL accounted for 74% and 26% of cases, respectively. Comorbidities included diabetes (18%), myocardial infarction (12%) and chronic pulmonary disorder (12%).
Following Induction 1, 25/33 evaluable patients (76%) achieved CR. Of these, 21 were evaluable for MRD: 19% reached a molecular CR, 14% had a low positive MRD, 67% had molecular failure, and three patients were not evaluable for MRD. Treatment failure and early death occurred in 9% and 6% of patients, respectively.
Following blinatumomab induction, 24/29 evaluable patients (83%) reached a CR. Of these, 21 were evaluable for MRD: 76% reached a molecular CR, 14% had a low positive MRD, 10% had molecular failure, and two patients were not evaluable for MRD. Treatment failure/relapse and early death occurred in 10% and 7% of patients, respectively.
The 1-year OS was 85%. The 1-year OS of patients with common pre-B-ALL had a better trend towards survival compared with patients with pro-B-ALL (89% vs 75%, respectively). Patients aged 55–65 years had an OS of 100% at 1 year, whereas patients aged >65 years had an OS rate of 66%.
Safety
Blinatumomab was well tolerated, and no death occurred during blinatumomab induction. Two deaths occurred in CR: one due to hemophagocytic lymphohistiocytosis and one with arterial disease. The patient with hemophagocytic lymphohistiocytosis died during consolidation but an association with prior blinatumomab treatment is possible.
Conclusion
This study demonstrated that blinatumomab in sequence with low-intensity induction chemotherapy was mostly tolerable and effective, with high response rates and low mortality. These data hold promise for an alternative treatment option for older patients with B-ALL; however, as highlighted for the previous studies, further follow-up with a larger patient pool is required to establish the potential benefit of this regimen in all subtypes of B-precursor ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

