All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Inotuzumab ozogamicin and blinatumomab in older adults with newly diagnosed B-ALL: ALLIANCE trial
Older patients (≥60 years) with newly diagnosed Philadelphia chromosome-negative (Ph-negative) acute lymphoblastic leukemia (ALL) have poor survival with conventional chemotherapy. The phase II ALLIANCE A042001 (NCT05303792) trial investigated induction with inotuzumab ozogamicin (InO) and consolidation with blinatumomab in older adult patients with relapse/refractory (R/R) ALL. The study hypothesized that this regimen would improve 1-year event-free survival (EFS) compared with historical outcomes with conventional chemotherapy (1-year EFS, 10%). The aim was to provide an alternative treatment to conventional chemotherapy as first-line therapy in older patients (≥60 years old) who may experience multiagent chemotherapy regimens as toxic and only modestly effective.
Study design
This phase II, interventional clinical trial enrolled patients who were previously untreated for CD22+ Ph-negative ALL. Patients eligible for this study had Ph-negative B-ALL and were
- ≥60 years of age;
- had an Eastern Cooperative Oncology Group Performance Status of 0 to 2;
- had no planned allogeneic or autologous hematopoietic cell transplant (HCT);
- had no active central nervous system or testicular leukemia or additional malignancy active within the previous 2 years; and
- had no history of chronic liver disease, veno-occlusive disease/sinusoidal obstruction syndrome, significant cardiac disease, or clinically relevant neurologic disorder.
The study consisted of a treatment schema involving two courses of InO given at an induction dose on a weekly basis before a response assessment. Patients who showed response after the first assessment went on to receive a second dose of InO and those who did not went straight onto consolidation therapy (Figure 1).
The primary endpoint was estimated 1-year EFS (event = progression of disease prior to the end of cycle 2 of consolidation blinatumomab, relapse, or death from any cause). Secondary endpoints included overall survival, relapse-free survival, complete response rate, minimum residual disease (MRD) negativity rate, treatment-related mortality, and safety and tolerability.
Figure 1. Patient treatment schema for ALLIANCE A042001*
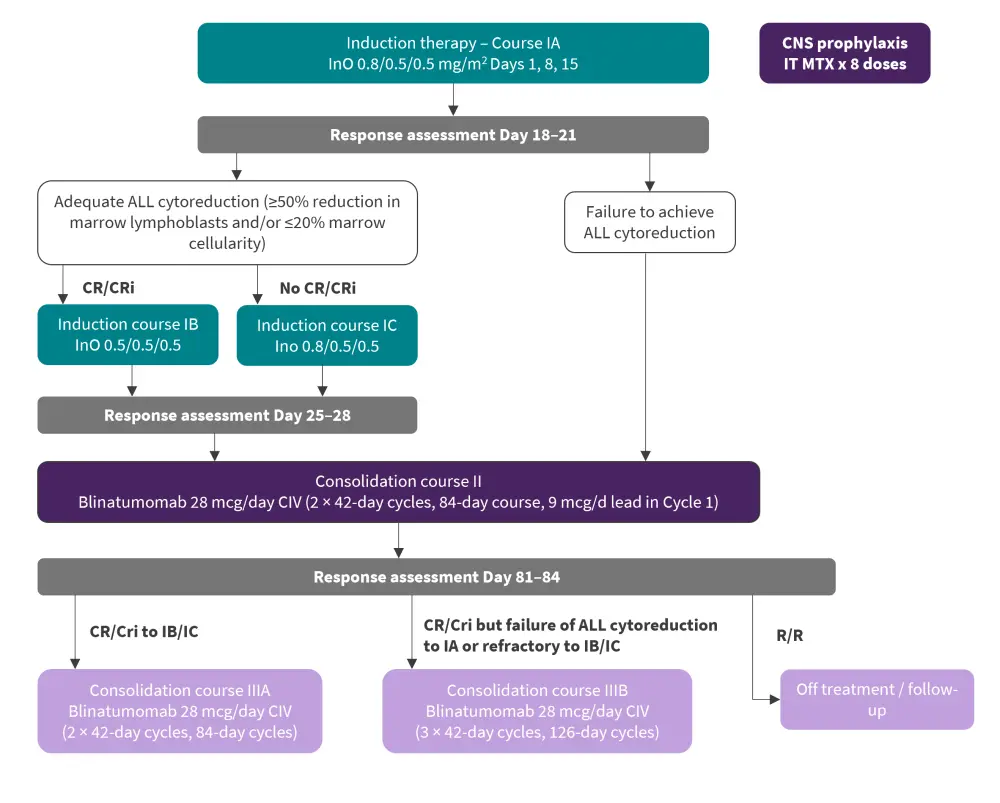
ALL, acute lymphoblastic leukemia; CIV, continuous intravenous infusion; CNS, central nervous system; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; InO, inotuzumab ozogamicin; IT MTX, intrathecal methotrexate; R/R, relapsed/refractory.
*Adapted from Wieduwilt, et al.1
Results
A total of 33 patients aged between 60–84 years were treated. At baseline, 24% had prior malignancy and chemo/radiotherapy and 18% had multiple myeloma. Additionally, 92% had CD22 expression. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
ECOG, Eastern Cooperative Oncology Group; WBC, white blood count; RXT, radiotherapy. |
|
|
|
Characteristic, % (unless otherwise stated) |
Total (N = 33) |
|
|---|---|---|
|
Age |
|
|
|
Median, years (range) |
71 (60-84) |
|
|
≥70 years |
52 |
|
|
Race |
|
|
|
White |
85 |
|
|
Asian |
3 |
|
|
Not reported / unknown |
12 |
|
|
Ethnicity |
|
|
|
Not Hispanic or Latino |
82 |
|
|
Hispanic or Latino |
9 |
|
|
Unspecified |
9 |
|
|
Gender |
|
|
|
Male |
58 |
|
|
ECOG performance status |
|
|
|
0 |
24 |
|
|
1 |
58 |
|
|
2 |
18 |
|
|
Prior malignancy and chemo-RXT |
|
|
|
Any |
24 |
|
|
Multiple Myeloma |
18 |
|
|
Presenting WBC (range), median ×1000/mcl |
3.2 (0.6–38) |
|
|
Median CD22 expression (range) |
92 (21–100) |
|
Hematologic response and efficacy
The study met its primary endpoint and overall survival after one year was promising, with median overall survival not been reached (95% confidence interval [CI], 31 months–not reached) (Figure 2). In total, 12 events were recorded in all patients, with nine patients relapsing from the systemic central nervous system, CD19 or CD22 negative.
Composite complete remission was achieved by 96% of patients in both treatment groups, with 60% of this group achieving complete responses. Complete response/complete remission with partial hematologic recovery/complete remission with incomplete hematologic recovery was achieved by 85% of patients on the course of treatment IA/IB/IC and CR was achieved by 58% of patients by the end of the second course of treatment.
Figure 2. Efficacy outcomes*
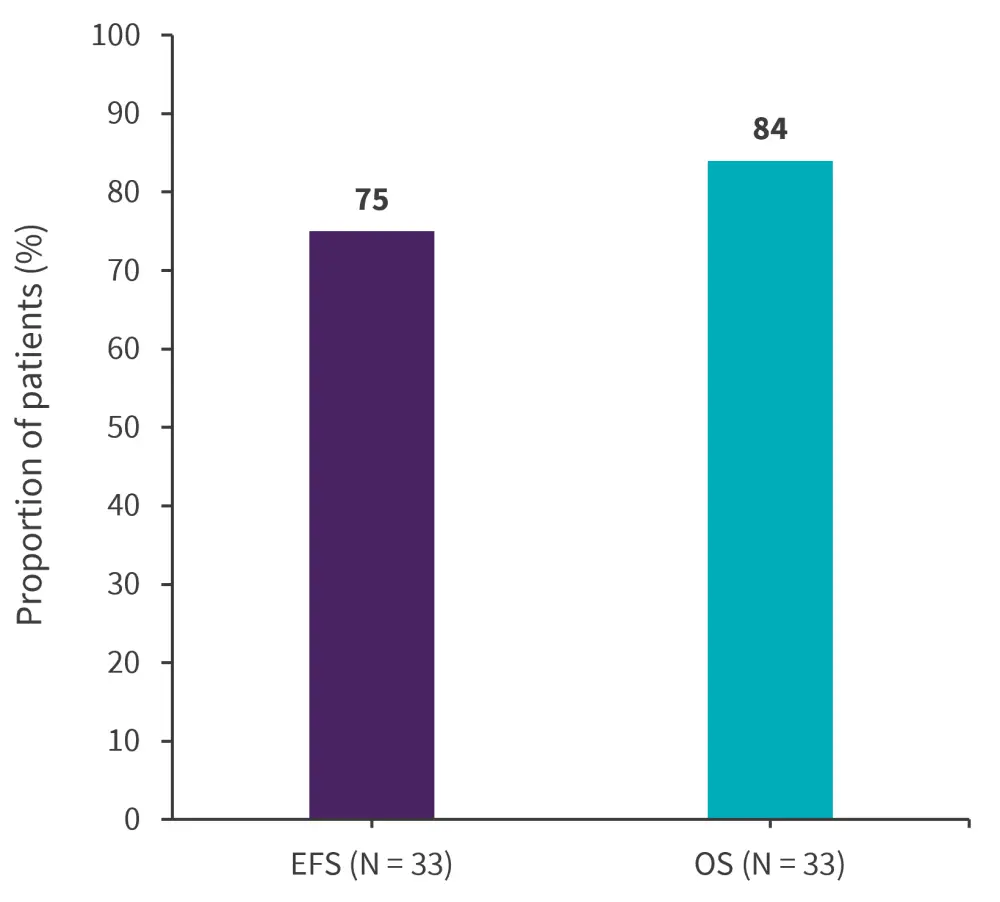
EFS, event-free survival; OS, overall survival
*Adapted from Wieduwilt, et al.1
Safety
The most common ≥Grade 3 adverse events reported in over 30% of patients were decreased neutrophil count (87.9%), platelet count decrease (72.7%), anemia (42.4%), and white blood cell decrease (39.4%). Overall, there were nine deaths, six due to relapsed ALL and three due to adverse events.
Conclusion
The ALLIANCE A042001 trial reached its primary endpoint. The author concluded that a conventional chemotherapy-free regimen of InO induction and blinatumomab consolidation was highly effective and safe in older patients with newly diagnosed Ph-negative CD22+ B-ALL. Further study is required to establish the significance of these results; however, this trial shows InO plus blinatumomab may be a more suitable alternative to conventional chemotherapy in this population of patients.
Your opinion matters
In your experience, which treatment goals are most important for older adult patients with acute lymphoblastic leukemia?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


