All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Novel targeted and cellular therapies in adult patients with R/R Ph-like ALL
Philadelphia (Ph)-like acute lymphoblastic leukemia (ALL), a high-risk subtype of B-cell ALL (B-ALL), is highly prevalent in Latinos and occurs in up to 20% of adults with newly diagnosed B-ALL.1 Genetic subgroups of Ph-like ALL are collectively characterized by a similar gene expression profile to Philadelphia-positive ALL but lack the BCR-ABL1 gene fusion. In this subtype, CRLF2 gene rearrangements and gene fusions are commonly detected with either IGH or P2RY8; less frequent gene fusions involve ABL-class genes, EPOR and JAK2.1
Compared with other B-ALL subtypes, patients with Ph-like ALL yield inferior outcomes when treated with conventional chemotherapy regimens, including higher rates of induction failure, minimal residual disease (MRD) persistence, and relapse rates; thus, there is an urgent need for treatment options for this patient population.1
Introduction of the novel immunotherapies, inotuzumab ozogamicin (InO), blinatumomab, and CD19 chimeric antigen receptor (CAR) T-cell therapy, has dramatically changed the treatment landscape of relapsed/refractory (R/R) B-ALL, each demonstrating promising single-agent activity.1 However, little is known about their impact on patients with R/R Ph-like ALL.1
The ALL Hub previously reported a summary of treatment strategies for Ph-like ALL. Below, we summarize a recent article by Aldoss et al.1 on the treatment outcomes of adult patients with R/R Ph-like ALL treated with novel immunotherapies.
Study design
This was a single-center retrospective study including adult patients with R/R Ph-like ALL who completed at least one treatment cycle of a novel salvage therapy (InO, blinatumomab, and/or CD19 CAR T-cell therapy), and were evaluable post-treatment.
The primary endpoint was the complete remission (CR) or CR with incomplete count recovery (CRi) rate.
Secondary endpoints included rates of MRD negativity, defined as achieving <0.01% leukemic cells by multicolor flow cytometry, the proportion of responders proceeding to allogeneic hematopoietic cell transplantation (allo-HCT) consolidation in remission, event-free survival (EFS), and overall survival (OS).
Results
Baseline characteristics
Overall, 96 patients were included in this analysis, 57% of whom received a single novel therapy and 43% who received >1 novel therapy, with a total of 149 novel therapies administered. The sequence of novel therapies by the line of therapy is highlighted in Figure 1. Patient characteristics across the different types of novel therapies are summarized in Table 1.
Figure 1. Sequence of novel therapies in study cohort*
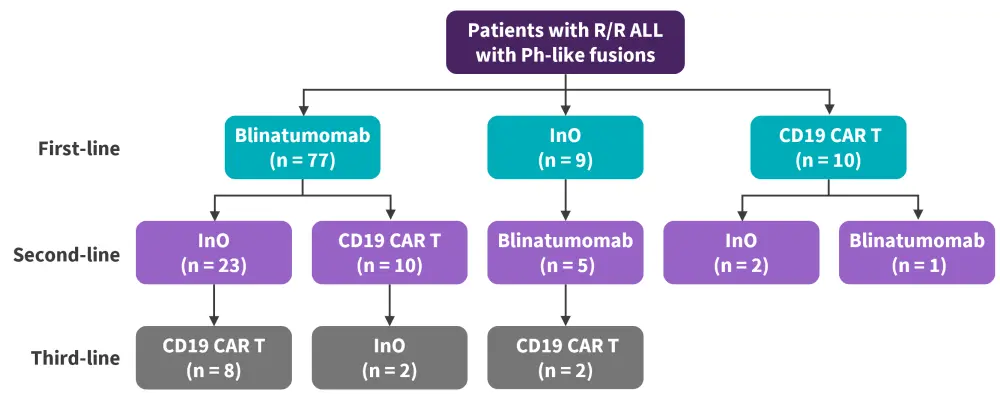
ALL, acute lymphoblastic leukemia; CAR, chimeric antigen receptor; InO, inotuzumab ozogamicin; Ph, Philadelphia; R/R, relapsed/refractory.
*Adapted from Aldoss, et al.1
Table 1. Patient characteristics*
|
Allo-HCT, allogeneic hematopoietic stem cell transplant; BM, bone marrow; CAR, chimeric antigen receptor; EMD, extramedullary disease; InO, inotuzumab ozogamicin; NA, not available; Ph-like, Philadelphia chromosome-like. |
|||||
|
Characteristic, % (unless otherwise stated) |
Total cohort |
Blinatumomab |
InO |
CD19 CAR T-cell |
p value |
|---|---|---|---|---|---|
|
Median age (range), years |
36 (18–71) |
36 (18–71) |
33 (19–71) |
29 (18–66) |
0.004 |
|
Sex |
|
|
|
|
0.97 |
|
Male |
69 |
67.5 |
69.4 |
66.7 |
|
|
Female |
31 |
32.5 |
30.6 |
33.3 |
|
|
Race |
|
|
|
|
0.50 |
|
Latinos |
88.5 |
88 |
94 |
93 |
|
|
White |
5 |
6 |
3 |
3 |
|
|
Black |
2 |
2 |
3 |
0 |
|
|
Asian |
4 |
4 |
0 |
3 |
|
|
Ph-like group |
|
|
|
|
NA |
|
CRLF2-IGH |
50 |
51 |
56 |
53 |
|
|
CRLF2-P2RY8 |
27 |
26.5 |
28 |
23 |
|
|
ABL-fusion |
8 |
7 |
3 |
13 |
|
|
JAK-fusion |
9 |
10 |
11 |
10 |
|
|
EPOR-fusion |
4 |
5 |
0 |
0 |
|
|
ETV6-NTRK3 fusion |
1 |
1 |
3 |
0 |
|
|
Median lines of therapy (range), n |
|
1 (1–6) |
2 (1–6) |
3 (1–7) |
<0.001 |
|
Median BM blasts (range) |
|
50 (0–95) |
80 (0–100) |
60 (0–97) |
0.065 |
|
EMD ± BM |
|
0 |
6 |
27 |
<0.001 |
|
Prior allo-HCT |
|
12 |
22 |
57 |
0.002 |
|
Prior novel therapy |
|
|
|
|
NA |
|
Blinatumomab |
|
NA |
69 |
70 |
|
|
InO |
|
6 |
NA |
33 |
|
|
CD19 CAR T cells |
|
1 |
11 |
NA |
|
Response rates
The remission and MRD-negativity rates achieved in evaluable patients across the different types of novel therapies are seen in Figure 2. Among patients responding to blinatumomab, InO, and CD19 CAR T-cell therapy, 50%, 50%, and 44% proceeded to allo-HCT consolidation, respectively.
Figure 2. CR/CRi rates across types of novel targeted and cellular therapies*
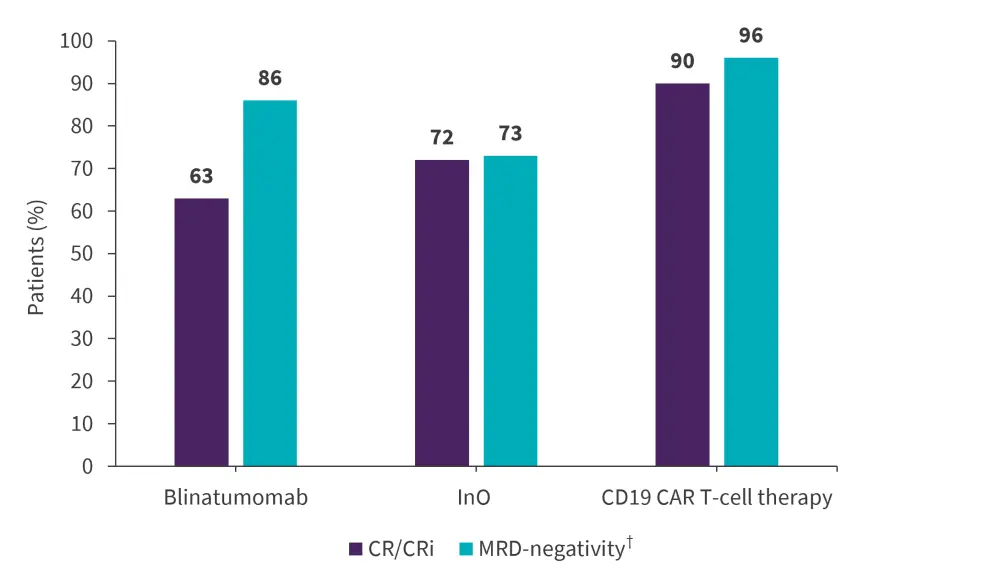
CAR, chimeric antigen receptor; CR, complete remission; CRi, complete remission with incomplete count recovery; InO, inotuzumab ozogamicin; MRD, minimal residual disease.
*Data from Aldoss, et al.1
†MRD-evaluable patients were n = 37 for blinatumomab; n = 22 for InO; and n = 30 for CD19 CAR T‑cell therapy
Moreover, in patients treated with blinatumomab, higher CR/CRi rates were achieved in patients who had low versus high disease burden (65% and 49%, respectively). For patients receiving InO with versus without prior novel therapies, the CR/CRi rates were 74% and 67%, respectively. Similarly, in patients treated with CAR T-cell therapy with or without prior novel therapy (blinatumomab or InO), the CR/CRi rates were 90% and 89%, respectively.
Multivariate analyses showed that both the type of therapy administered and pretreatment bone marrow blasts were predictive of response rates; lower CR/CRi rates were reported in patients treated with blinatumomab compared with InO and CD19 CAR T-cell therapy (p = 0.044), as well as in those with higher pretreatment marrow blasts (p = 0.024). Conversely, the number of prior lines of therapy (p = 0.19), age (p = 0.47), prior allo-HCT (p = 0.62), and Ph-like subgroups (p = 0.74) did not significantly impact disease response.
Survival outcomes
The median OS for the total cohort was 21.6 months. The 12-month OS for patients with CRLF2-IGH, CRLF2-P2RY8, and other non-CRLF2 fusion types after the first-salvage therapy were 61%, 79%, and 70%, respectively (p = 0.04).
In multivariate analyses, pretreatment marrow blasts (p = 0.022) and the Ph-like fusion subgroup (p = 0.016) were independently predictive of EFS outcomes; the 12-month EFS for IGH-CRLF2, P2RY8-CRLF2, and other non-CRLF2 fusions was 22%, 34%, and 48%, respectively. Moreover, post-allo-HCT consolidation response was significantly associated with a higher EFS (p < 0.001), with a 12-month EFS of 72% vs 15% for responders who did versus did not undergo allo-HCT consolidation, respectively.
However, the type of novel therapy used (p = 0.19) did not impact EFS outcomes, with a 12-month EFS of 29%, 25%, and 41% for blinatumomab, InO, and CD19 CAR T responders, respectively. Similarly, age (p = 0.81), prior lines of therapy (p = 0.73), and prior allo-HCT (p = 0.88) did not significantly impact EFS.
Relapse patterns
Overall, there were 26 relapses in blinatumomab (18 isolated bone marrow disease, two isolated central nervous system [CNS], five isolated non-CNS extramedullary disease, and one combined bone marrow), 17 relapses In InO (ten isolated bone marrow disease, two isolated CNS, two isolated non-CNS extramedullary disease, two combined bone marrow and CNS, and one combined bone marrow and non-CNS EMD), and 11 relapses in CD19 CAR T-cell therapy (eight isolated bone marrow relapses, two isolated non-CNS extramedullary disease, and one isolated CNS).
Conclusion
This single-center retrospective analysis demonstrated that novel therapies, such as blinatumomab, InO, and CD19 CAR T-cell therapy, can induce high MRD-negative remission rates in adult patients with R/R Ph-like ALL and facilitate subsequent allo-HCT consolidation in a significant proportion of patients. Future studies focusing on the early use of these novel therapies are likely to further improve treatment outcomes for this high-risk subgroup.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


