All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Management of CNS disease in adults with ALL
Central nervous system (CNS) disease at the time of diagnosis occurs in a minority of adult patients with acute lymphoblastic leukemia (ALL), with an incidence of 5–11% within the largest clinical trials over the last 25 years; CNS disease was also associated with inferior overall survival rates in the MRC UKALL12/ECOG 2993 trial. The standard use of CNS-directed prophylactic measures in current ALL treatment protocols have reduced CNS relapse rates; however, some patients still experience relapse, either in isolation or concurrent with other sites, which has been linked to poor outcomes.1
Below, we summarize an article by Kopmar et al.1 on the management approaches of CNS disease in adults with ALL for common and challenging clinical scenarios, including data derived from some pediatric studies. The areas covered include risk factors, diagnostic tools, prevention, and treatment techniques.
Diagnostic approaches and risk factors in CNS disease1
Risk factors
A high white blood cell count (>30,000/µL in B-cell ALL and >100,000/µL in T-cell ALL); lactate dehydrogenase (LDH) >3 times the upper-limit-of-normal (ULN), adverse-risk cytogenetics (including t[9;22] and t[4;11]), T-lineage, and history of CNS disease have been linked to CNS relapse risk.
Diagnostic approaches in CNS disease
Most cases of CNS involvement in adult ALL are asymptomatic or mildly symptomatic, with variable neurological symptoms dependent on the neuroanatomic structures affected. The diagnostic tools in CNS disease involve CNS neuroimaging and cerebrospinal fluid (CSF) sampling via lumbar puncture, enabling microscopic examination for conventional cytology and/or flow cytometry.
CNS imaging
Magnetic resonance imaging is typically carried out in patients presenting with neurological symptoms or signs. Examples of clinical presentations with CNS disease include cranial nerve infiltration, leptomeningeal involvement, and parenchymal lesions; elevated intracranial pressure/CNS hemorrhage can occur in rarer cases.
Cerebrospinal fluid (CSF) examination
In usual clinical practice, lumbar punctures (LPs) are carried out around the time of the first scheduled intrathecal (IT) chemotherapy, or earlier in instances of clinically presented CNS disease. Pediatric studies have revealed that CSF contamination in both first and second lumbar puncture samples is linked with inferior event-free survival (EFS) rates, justifying the use of IT chemotherapy prior to establishing CNS disease status. For adults in this practice, however, postponing initial LP testing until clearance of leukemic blasts in the CSF is the preferred approach.
Retrospective studies suggest that platelet counts of <50,000/ µL were associated with an increased risk of traumatic LPs and eventually inferior outcomes in children. Hence, correction above 50,000/µL is the recommended platelet threshold during LPs, with a similar practice for the management of coagulopathy.
Although conventional cytospin (CC) has historically been used for microscopic CSF examinations, multi-flow cytometry (MFC) has demonstrated a higher sensitivity for detecting CNS leukemia and greater risk-stratification for CNS relapse following treatment in recent studies. A grading system of CNS involvement has been characterized by the Children’s Oncology Group (Figure 1).
Figure 1. CNS involvement classification*
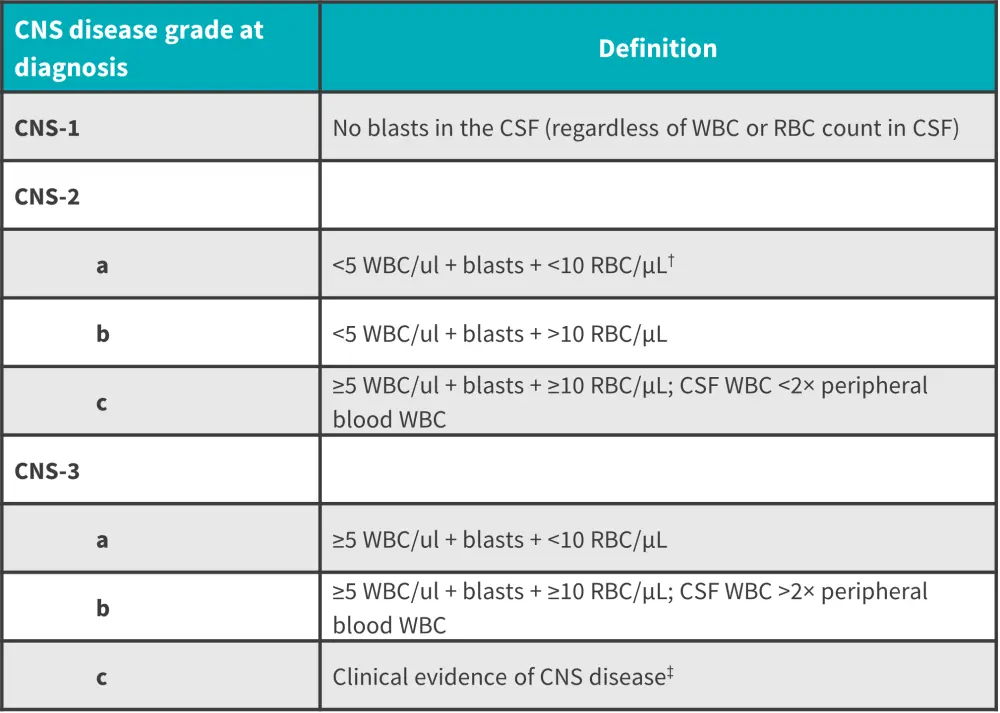
CNS, central nervous system; CSF, cerebrospinal fluid; RBC, red blood cell; WBC, white blood cell.
*Data from Kopmar and Cassaday.1
†Criteria for a traumatic LP.
‡Includes cranial nerve palsies or other overt neurologic deficits not attributable to other causes.
Prevention and treatment techniques in CNS disease1
Strategies include CNS-penetrating systemic agents and IT chemotherapy. Additional options for active CNS disease, both at diagnosis or relapse, may include radiotherapy (RT), CD19 chimeric antigen receptor T-cell (CAR-T), and hematopoietic cell transplantation (HCT). Based on four different clinical scenarios of CNS involvement, the authors devised several treatment-based approaches; the CNS-directed therapies for each case are summarized in Figure 2.
Figure 2. CNS-directed therapies at different stages of CNS involvement*
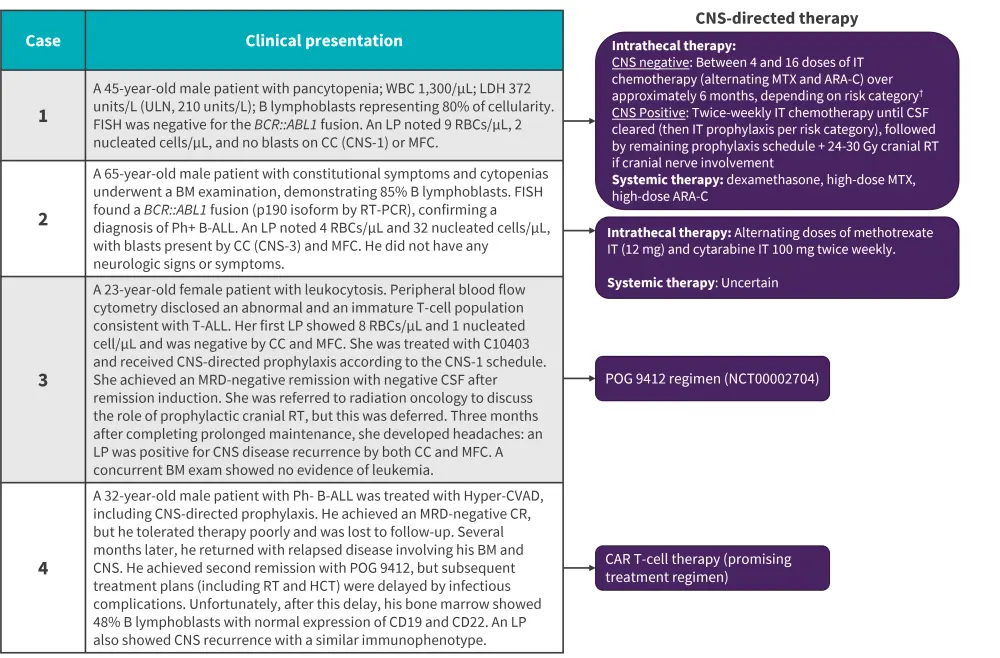
Ara-C, cytosine arabinoside; ALL, acute lymphoblastic leukemia; BM, bone marrow; CAR T-cell, chimeric antigen receptor; CC, conventional cytospin; CNS, central nervous system; CR, complete response; CSF, cerebrospinal fluid;; FISH, fluorescence in situ hybridization; HCT, hematopoietic stem cell transplant; Hyper-CVAD, hyper fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine; IT, intrathecal; LDH, lactate dehydrogenase; LP, lumbar puncture; MFC, multi-channel flow cytometry; MRD, minimal residual disease; MTX, methotrexate; PCR, polymerase chain reaction; Ph+, Philadelphia-chromosome positive; RBC, red blood cell; RT, radiotherapy; ULN, upper limit of normal; WBC, white blood cell.
*Data from Kopmar and Cassaday.1
†Patients were considered high risk if the LDH was >600 units/L or proliferative index >14%.
Philadelphia-negative (Ph-) B-ALL without CNS involvement at diagnosis: Case 1
Hyper fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine (hyper-CVAD) is the standard regimen used, alongside variable schedules of IT therapy based on whether the patient is CNS-positive or negative (Figure 2).
CNS-penetrating systemic agents include methotrexate, cytarabine, dexamethasone, asparaginase, 6-mercaptopurine, and dasatinib, with high-dose methotrexate (HD-MTX) commonly used in many adult protocols. Within the COG-AALL0232 study (NCT00075725), HD-MTX in interim maintenance (5,000 mg/m2) has yielded higher EFS rates and reduced CNS relapse rates in children and young adults with B-ALL; however, in the parallel COG-AALL0434 (NCT00408005) study in T-ALL, Capizzi escalating-dose methotrexate (100–300 mg/m2) plus pegaspargase demonstrated higher disease-free survival and lower CNS relapses (0.4% vs 3%) when compared to HD-MTX; this suggests the use of multiple CNS-penetrating agents may be beneficial over HD-MTX.
Within adult ALL regimens, the use of these penetrative systemic agents is enhanced by other CNS-directed prophylaxis, all including IT methotrexate at varied doses and frequencies. A risk-stratified approach of Hyper-CVAD IT treatments (methotrexate and cytarabine), based on WBC and LDH factors, include 8 IT treatments in low-risk patients (neither WBC >30,000/µL nor LDH >3× ULN) versus 10 IT treatments in high-risk patients (both >30,000/µL nor LDH >3× ULN).
Although cranial RT was historically given alongside IT therapy for CNS-directed prophylaxis, there were concerns about delayed toxicities (neurocognitive defects, endocrinopathy, and secondary cancers) and data on its removal did not compromise CNS relapse rates. As a result of this, RT is currently reserved for CNS disease at diagnosis or relapse and in high-risk subgroups/features such as T-ALL.
Philadelphia-positive (Ph+) B-ALL with CNS involvement at diagnosis: Case 2
The standard IT approach is alternating doses of methotrexate and cytarabine (Figure 2). If CNS-negativity by MFC is achieved, the CNS-directed prophylaxis schedule for CNS-3 status then follows. If still CNS-positive, other CNS-directed therapy treatment alternative approaches such as ommaya reservoir, a surgically placed intraventricular catheter aimed to facilitate easier repeat of drugs into the CSF, can be considered. This device has demonstrated pharmacokinetic benefits and a 50% dose reduction in pediatric studies, it is typically only used when blasts remain in CSF after intensive IT and systemic therapy.
CNS-directed RT is a viable treatment option when patients present with cranial nerve deficits, parenchymal involvement, and persistent CSF disease despite enhanced intrathecal chemotherapy treatment. Factors that require careful consideration for the use of RT are the use of HCT, as this may impact the total radiation dose, and the potential risk of increased toxicity if IT chemo and RT are used in close proximity.
With the employment of newer ‘chemotherapy-free’ treatments in Ph+ ALL and the unknown efficacy of these strategies in CNS disease, the optimal systemic therapeutic regimen is uncertain. Dasatinib is the recommended tyrosine kinase inhibitor option in this setting, as it has demonstrated CNS penetrating activity in both preclinical and clinical settings. A recent childhood Ph+ B-ALL study showed a significantly lower 4-year cumulative CNS relapse risk for patients treated with dasatinib plus intensive chemo versus imatinib (2.7% vs imatinib 8.4%); similar rates were observed in a chemotherapy-free trial. Newer-generation tyrosine kinase inhibitors, such as ponatinib, have shown CNS activity in preclinical models achieving higher CSF concentrations compared to dasatinib; however, its CNS-specific efficacy is currently unknown.
HCT is an option in patients with Ph+ ALL regardless of CNS status which is historically given in first clinical remission. In this clinical case and other similar cases, it is a viable option if there is no CNS disease within the CSF. For older patients, reduced intensity conditioning is the common approach used, although several retrospective studies have shown higher relapse rates with this regimen when compared to myeloablative conditioning.
Isolated CNS relapse of T-cell ALL: Case 3
The three key considerations for patients with either isolated or multiple relapses include intensive CNS-directed therapy including IT and RT modalities, multi-agent systemic therapy with CNS-active agents, and consideration of HCT if the CSF clears.
Systemic therapies involving prolonged and intensive schedules of multi-agents (dexamethasone, asparaginase, and high-dose methotrexate and cytarabine) are employed within the pediatric setting. This approach is likely only applicable to adolescent and young adult populations with consideration of the Ommaya reservoir for frequent administration. For this clinical scenario, regimens such as POG 9412, using intensified CNS-directed systemic chemo can be used. Intra-CSF chemo can include intrathecal triple therapy and the risk of toxicities is justified in this setting.
The typical adult dose of RT is 24 Gy given in a fractionated dosing schedule. RT strategies that can be considered within this clinical setting are low-dose (usually 6 Gy) cranial boost prior to TBI which has improved post-HCT CNS-relapse-free survival and reduced toxicity; a proton-beam therapy may also reduce toxicity.
Given the patients young age, relapsed disease, and T-cell phenotype, HCT can be considered in this setting. There is no consensus approach but data from pediatric studies supports the delay of HCT in patients with isolated CNS relapse and favorable prognostic features without affecting treatment outcomes and its beneficial use for patients with early relapses and high-risk features (older age).
Multiply relapsed disease involvement of both bone marrow and CNS: Case 4
There is increasing interest in the role of novel immunotherapeutic agents, CAR T-cell therapies, and bispecific antibodies as potential strategies in the management of CNS disease.
CAR T-cell therapies have demonstrated efficacy and safety in recent trials. In a post-hoc analysis of five CD19-directed CAR T-trials that included children and adolescent/young adult patients with R/R B-ALL and a history of CNS involvement (n = 154), no difference in RFS, OS, or toxicity were found between those with versus without CNS disease. Another retrospective analysis in children and adolescents/young adults with active CNS disease at enrolment and treated with CDI9 CAR T-cells reported a complete response rate of 94%, although no effective mitigation on subsequent systemic or CNS relapse was observed. CAR T-cell therapy in CNS disease in the first prospective analysis demonstrated clearance of CNS disease in 85.4%, with similar survival and toxicity rates to previous standards.
There is limited data on the effect of blinatumomab and inotuzumab ozogamicin in CNS disease, owing to the lack of representation of this patient subset within clinical trials. In one retrospective report that included 11 patients with CNS-positive R/R B-ALL, blinatumomab was well-tolerated despite two cases of neurotoxicities; however, blinatumomab resulted in higher rates of CNS relapse in another study. To date, there is no known clinical evidence for the use of inotuzumab ozogamicin in CNS disease.
Conclusion
The management approaches for CNS disease in adult ALL depend on the clinical presentation and diagnostic work-up at the time of diagnosis or upon relapse. Current strategies for both CNS prophylaxis and treatment include CNS-directed IT and systemic-based therapies. The use of RT is a decreasing option for CNS prophylaxis. On the other hand, RT, consolidative HCT, and more recently CAR T-cell therapy, represent promising treatment options across different stages of active CNS disease. Future investigations into ways to further mitigate the risk of CNS relapse and new treatment strategies for high-risk patients are needed. More definitive trials are warranted to standardize the procedures and permit optimal management of CNS disease in adult patients with ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

