All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Nelarabine plus standard chemotherapy does not improve survival in adult patients with T-ALL: First analysis of UKALL 14 study
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive variant of ALL with very poor prognosis mainly affecting children, adolescents, and young adults (AYA). The treatment for AYA with T-ALL is based on pediatric protocol in the UK. Nelarabine is a DNA-terminating nucleoside used in the treatment of patients with T-ALL. Toxicity events related to the use of nelarabine as a single agent or in combination with chemotherapy have been previously reported in patients with T-ALL, particularly those with relapse/refractory T-ALL. However, a recent USA study, previously reported by the ALL Hub, has shown improved survival with the addition of nelarabine to standard chemotherapy for pediatric and young adult patients with T-ALL.
Here we summarize the key findings from the first analysis of the UKALL14 study (NCT01085617) presented during the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition. Rowntree, et al.1 presented findings from their analysis investigating the efficacy of nelarabine when added to standard chemotherapy in patients with T-ALL.
Study design
This was a phase III randomized controlled trial conducted in the UK between 2012 and 2018, in adult patients aged 25–65 years with newly diagnosed T-ALL. Patients with central nervous system disease were included at the start of the study; however, patients developing neurotoxicity Grade ≥2 before they were due to receive nelarabine were excluded. Eligible patients were randomized to standard of care (SOC) induction phase I and II chemotherapy with or without nelarabine. Patients in the SOC + nelarabine group received 1.5 g/m2 of nelarabine on Day 1, 3, and 5 following second phase of induction (Figure 1).
- The primary endpoint was 3-year event free survival (EFS), defined as relapse or death from any cause.
- Secondary endpoints included overall survival (OS), time to relapse, remission, and MRD negativity rates at end of phase 1 and 2 induction and toxicity.
An exploratory analysis was also conducted based on the hypothesis that most immature cases of T-ALL are detected through absence of biallelic T-cell receptor deletion (ABD), which is an adverse prognostic marker of T-ALL. In addition, early T-cell progenitors express high levels of SAMHD1, a gene recently shown to mediate resistance to nelarabine. The exploratory endpoint included association between ABD status and outcomes, and any differential effects of nelarabine according to ABD status.
Figure 1. Treatment schema*
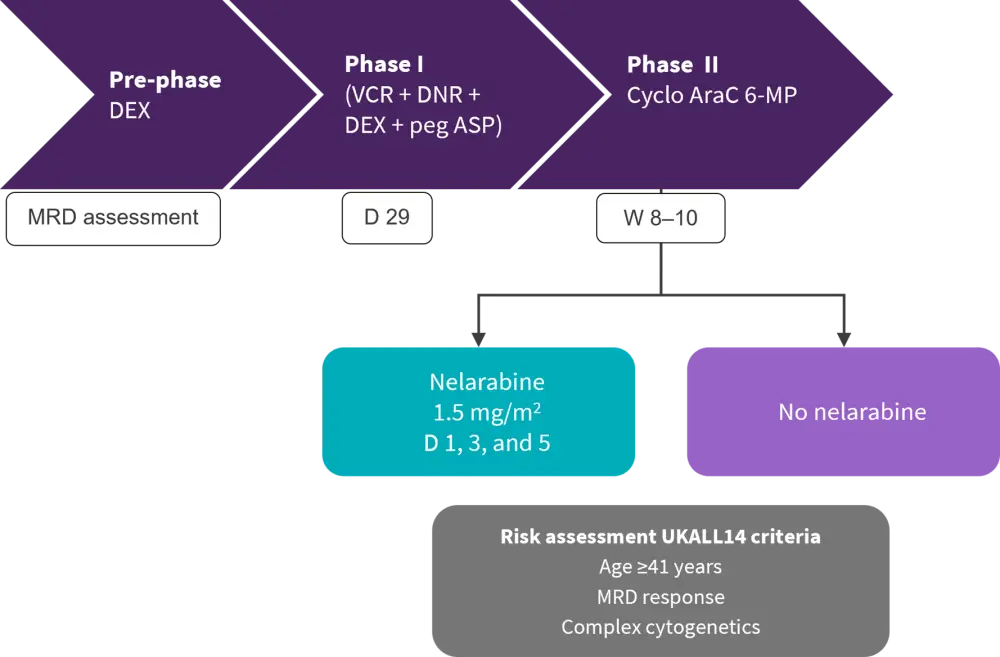
AraC, cytarabine; D, day; DEX, dexamethasone; DNR, daunorubicin; MRD, minimal residual disease; Peg ASP, pegaspargase; W, week; VCR, vincristine; 6-MP, 6-mercaptopurine.
*Adapted from Rowntree, et al.1
Results
Baseline characteristics
A total of 144 patients were included, 75 in SOC group and 69 in the SOC + nelarabine group, respectively. Twenty-one patients were not given nelarabine in the SOC + nelarabine group mainly due to persistent treatment-related toxicity (Figure 2).
Figure 2. Consort diagram*
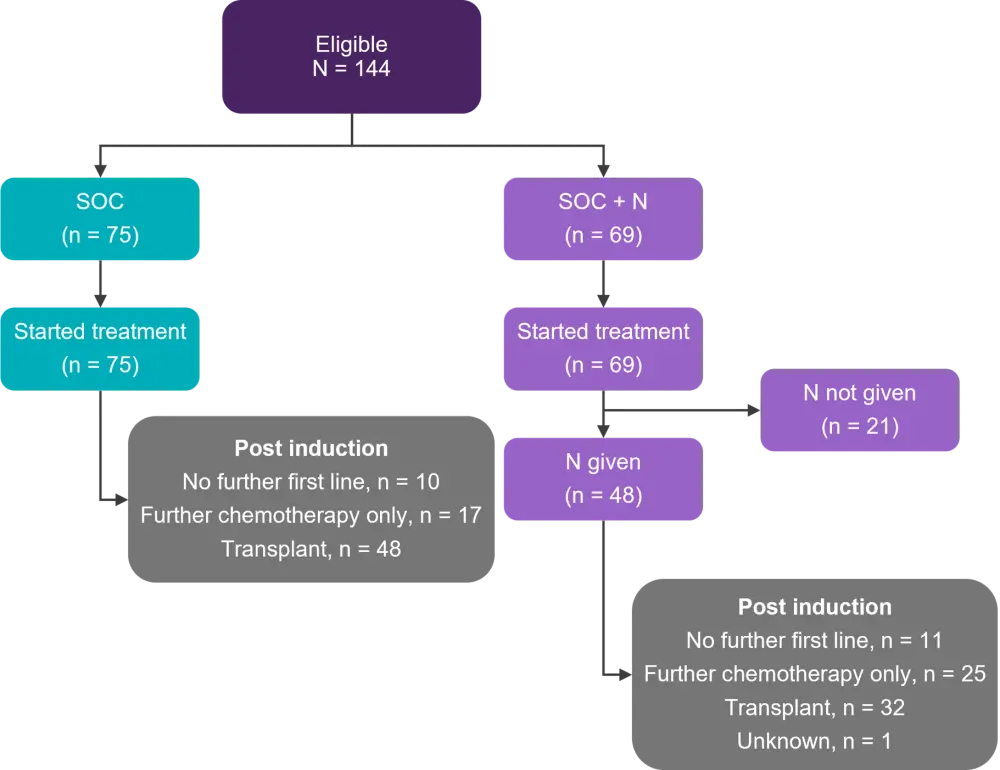
N, nelarabine; SOC, standard of care
*Adapted from Rowntree et al.1
The median age was 38 years (range, 25–61 years) and baseline characteristics were balanced between the two groups, except for a higher proportion of patients having complex cytogenetics in the SOC group compared with the SOC + nelarabine group (18% vs 4%; Table 1).
Table 1. Baseline characteristics*
|
CGN, cytogenetics; ECOG, Eastern Cooperative Oncology Group; N, nelarabine; SOC, standard of care; WBC, white blood cell. |
||
|
Characteristics, % (unless otherwise stated) |
SOC |
SOC + N |
|---|---|---|
|
Median age (range), years |
38 (25–61) |
36 (25–64) |
|
Male |
71 |
70 |
|
ECOG Performance Status |
|
|
|
0–1 |
95 |
93 |
|
≥2 |
5 |
7 |
|
WBC >100 × 109/L |
23 |
23 |
|
Complex karyotype |
18 |
4 |
|
Any UKALL14 CGN risk factor |
20 |
9 |
|
UKALL14 risk group |
|
|
|
Standard |
25 |
29 |
|
High |
64 |
61 |
Efficacy and safety
- The 3-year and 5-year EFS was 57% vs 61.7% and 54.1 vs 55.5% in the SOC vs SOC + nelarabine group, respectively (hazard ratio [HR], 0.88; p = 0.61).
- Similarly, 3-year and 5-year OS was 61.5% vs 65.7% and 59.1 vs 57.2% in the SOC and SOC vs SOC + nelarabine group, respectively (HR, 0.91; p = 0.73).
- A total of 39% vs 37% of deaths occurred in the SOC vs SOC + nelarabine group, respectively.
- Including 10.7% vs 7.3% of deaths occurring in first remission in the SOC and SOC + nelarabine group, respectively.
- Relapse was the most common cause of death in both the SOC and SOC + nelarabine group (28.0% vs 26.9%, respectively).
Exploratory analysis
A total of 108 patients were included in the exploratory analysis, of which 11, 54, and 55 had indeterminate, ABD, and non-ABD status, respectively (Table 2).
Patients with ABD status were:
- Older compared to patients with non-ABD status (median age 43.5 vs 34 years) (Table 2);
- Less likely to be MRD negative post-induction (p < 0.001) compared to non-ABD patients (Table 2); and
- Had significantly higher relapse rate at 3 years (42.7% vs 16.0%) compared to non-ABD patients.
ABD status retained prognostic value in multivariate analysis, however there was no interaction with the treatment arm (p = 0.46).
Table 2. Baseline characteristics, responses, and outcomes in patients with ABD*
|
ABD, absence of biallelic T-cell receptor deletion; CR, complete response; MRD, minimal residual disease; SCT, stem cell transplantation. |
|||
|
Characteristics, % (unless otherwise stated) |
Non-ABD |
ABD |
p value† |
|---|---|---|---|
|
Median age (range), years |
34 (25–60) |
43.5 (25–64) |
0.056 |
|
Age ≥41 years |
30.9 |
55.6 |
0.009 |
|
Male |
81.8 |
66.7 |
0.070 |
|
UKALL14 cytogenetic |
14.6 |
16.2 |
0.85 |
|
Extramedullary |
83.6 |
72.2 |
0.15 |
|
Any high-risk factor at |
56.4 |
75.9 |
0.031 |
|
Responses |
|||
|
Phase I CR |
92.7 |
81.5 |
0.081 |
|
Phase I MRD negative CR |
41.8 |
9.3 |
<0.001 |
|
Phase II CR |
94.6 |
90.7 |
0.45 |
|
Phase II MRD negative CR |
58.2 |
24.1 |
<0.001 |
|
No MRD target identified |
1.8 |
42.9 |
<0.001 |
|
Outcomes |
|||
|
No further first line of treatment |
12.7 |
16.7 |
— |
|
SCT |
54.6 |
57.4 |
— |
|
Chemotherapy alone |
32.7 |
24.1 |
— |
Conclusion
This phase III study demonstrated that three doses of nelarabine added to standard chemotherapy were well tolerated in adult patients with T-ALL. However, this did not translate into improved EFS or OS benefit. In comparison to historical trials, survival outcomes were improved in the UKALL14 trial, despite the older age of patients. ABD was identified as an independent negative prognostic factor in patients with de novo T-ALL and patients with ABD status were at higher risk of relapse. The study could not demonstrate the efficacy of nelarabine based on ABD status; therefore, further prospective studies are warranted to investigate the correlation between ABD status and outcomes in patients with T-ALL treated with nelarabine.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

