All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Novel treatment approaches in Ph+ ALL: Key updates from ASH 2022
BCR-ABL is a key biomarker of Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL), a subtype that most commonly occurs in adult patients. Historically, Ph+ ALL has been associated with poor prognosis; however, the introduction of tyrosine kinase inhibitors (TKIs) has significantly improved the treatment landscape for this patient subset. Third-generation BCR-ABL TKIs have exhibited potent activity; as illustrated by ponatinib’s activity against the 944C→T (Thr315Ile) mutation, which is implicated in treatment resistance and relapse,1,2 and olverembatinib’s promising activity in patients with chronic myeloid leukemia (CML) and Ph+ ALL.3
In this article, we summarize five key trials of TKI-based regimens for adult patients with Ph+ ALL, presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition.
Olverembatinib in patients with heavily pretreated/refractory chronic myeloid leukemia and Ph+ ALL3
Results from a U.S. based phase Ib study (NCT04260022) of olverembatinib in patients with CML and Ph+ ALL who experienced resistance or intolerance to at least two prior TKIs, including ponatinib, was presented by Elias Jabbour.3 This study was conducted based on the demonstrated activity of olverembatinib in patients with CML within China. The primary endpoint was pharmacokinetics and secondary endpoints were efficacy and safety.
Results
Overall, 51 patients were enrolled including 38 with CML-chronic phase (CP) and 13 with advanced stage Ph+ leukemia (blast phase (BP), accelerated-phase, and Ph+ ALL). Prior ponatinib therapy did not yield a response in 28 patients, due to resistance and intolerance in 21 and 7 patients, respectively; 19 of patients harbored the T315L mutation.
Pharmacokinetic profile
Olverembatinib exhibited dose proportionality, with an approximate increase in its systemic plasma exposure from 30 mg to 50 mg every other day. The maximum concentration range was 14.8–32.9 ng/ML, median Tmax was 4–6 hours, and half-life was 17.7–20.9 hours. No significant drug accumulation occurred even after multiple administrations. This pharmacokinetics data is comparable to historical controls from the Chinese-based study.
Efficacy
Overall, olverembatinib achieved efficacious responses in evaluable CML-CP patients (n = 23) across dose, mutation status, and in those who failed ponatinib; response was also observed within the evaluable advanced Ph+ leukemia cohort (n = 7; Figure 1).
Figure 1. Response rates in patients with CML and Ph+ ALL*
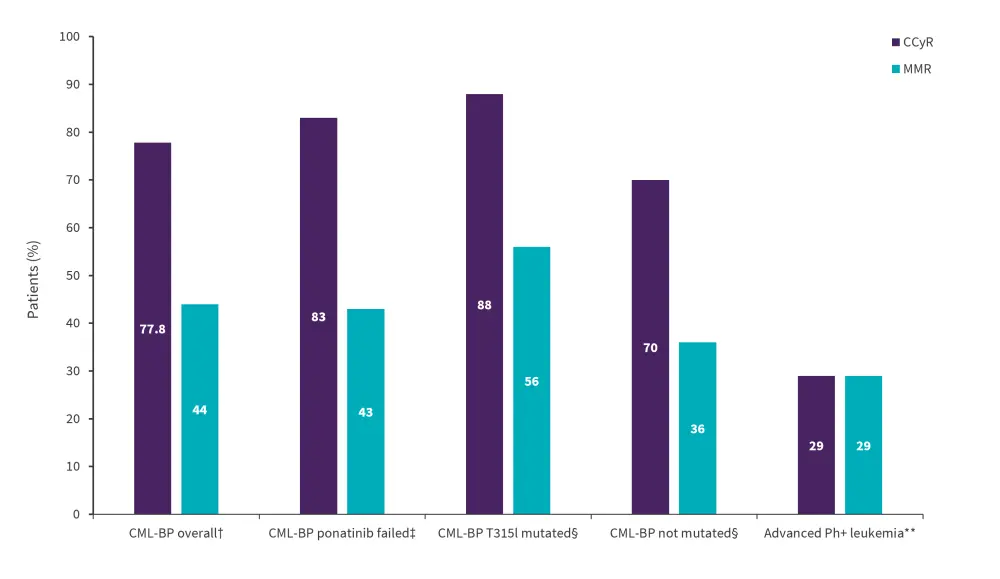
CCyR, complete cytogenetic response CML-BP, chronic myeloid leukemia-blast phase; MMR, major molecular response; Ph+, philadelphia chromosome-positive.
*Adapted from Jabbour.3
†n = 14/18 for CCyR; n = 10/23 for MMR.
‡n = 10/12 for CCyR; n = 6/14 for MMR.
§Mutated (n = 7/8 for CCyR; n = 5/9 for MMR) and non-mutated (n = 10/12 for CCyR and n = 6/14).
**n = 2/7 for both CCyR and MMR.
- Both responders (one with Ph+ ALL and one with accelerated-phase CML) in the advanced Ph+ group did not harbor the T315l mutation and were resistant to prior ponatinib treatment.
- At a median follow-up of 9 months, a complete cytogenetic response and major molecular response was achieved within 14 and 24 weeks in the CML-BP group and within 1.5 months and 24 weeks in the advanced Ph+ leukemia group, respectively.
Safety
The incidence of hematological toxicities (thrombocytopenia, neutropenia, and leukopenia) was lower compared with the study from China. Other treatment-related adverse events (AEs) include increased blood creatinine phosphokinase (during Cycle 1; 35%), increased aminotransferases (24%), increased lipases (19%), and fatigue (26%). Serious AEs were reported in six patients, none of which resulted in treatment discontinuation.
Presenter’s conclusion
Olverembatinib showed promising results in patients with CML-CP and advanced Ph+ ALL following treatment with ponatinib, including those with or without T1315l mutations.
Ponatinib plus blinatumomab in patients with newly diagnosed Ph+ ALL4
Data from the phase II substudy analysis of ponatinib plus blinatumomab in newly diagnosed patients with Ph+ ALL (NCT03263572) were presented by Short. The primary endpoint was complete molecular response (CMR) rate, secondary endpoints were event-free survival, overall survival, and safety.
Results
Baseline characteristics have been previously reported on the ALL Hub. Of the 60 patients enrolled, 40 had newly diagnosed Ph+ ALL; 60% had ≥1 cardiovascular-related risk factors and median CD19 expression was 99.8%.
Response rates and survival rates were evident in the evaluable cohort at the median follow-up of 18 months (Table 1). During Cycle 1, 58% had achieved a complete response (CR) in the peripheral blood with a rapid reduction of the BCR/AB1 transcript after 2 weeks of treatment.
Table 1. Response and survival rates*
|
ALL, acute lymphoblastic leukemia; CR, complete response, CRi, complete response with incomplete hematologic recovery; EFS, event-free survival; MRD, minimal residual disease; NGS, next-generation sequencing; ORR, overall response rate; OS, overall survival; Ph, Philadelphia-chromosome. |
|
|
Response, % (unless otherwise stated) |
Newly diagnosed Ph+ ALL (n = 40) |
|---|---|
|
Hematologic responses† |
|
|
ORR |
96 |
|
CR |
93 |
|
CRi |
4 |
|
Early death |
3 |
|
Complete molecular response‡ |
|
|
Overall |
87 |
|
After cycle one |
68 |
|
NGS MRD negative |
88 |
|
Survival analysis |
|
|
2-year EFS |
92 |
|
2-year OS |
95 |
Among the newly diagnosed cohort, there was one death in CR, one early death, and two relapses; one patient received hematopoietic stem cell transplant (HSCT) in CR1 and the remaining patients are in ongoing remission without HSCT.
Safety
There were no Grade 4 or higher treatment-related AEs reported. Ponatinib plus blinatumomab-related Grade 3 AEs included increased ALT, in four patients; pancreatitis, in two; and rash, hypertension, atrial fibrillation, febrile neutropenia, and coronary artery stenosis in one patient each. Discontinuations due to AEs occurred in three patients.
Presenter’s conclusion
Ponatinib plus blinatumomab proved to be efficacious and safe in newly diagnosed patients with Ph+ ALL, with deep, early, and durable responses observed without the need for HSCT. Follow-up analyses are necessary to further evaluate the risk of late relapses.
Hyper-CVAD plus ponatinib in patients with newly diagnosed Ph+ ALL: 6-year follow-up results5
The 6-year follow-up results of hyper-CVAD plus ponatinib in patients with newly diagnosed Ph+ ALL (NCT01424982),recently published in American Journal of Hematology,6 were presented by Nasnas. The study endpoints included efficacy (CR, cytogenetics and molecular); CR duration; survival outcomes; and safety.
Results
Among the 86 patients who were enrolled and treated, 66 were previously untreated and 20 were previously treated; of these 20 patients, there were two non-responders and 18 in CR. CR was achieved within four weeks of treatment (Table 2).
Table 2. Response rates in evaluable patients*
|
ALL, acute lymphoblastic leukemia; CcyR, complete cytogenetic response; CR, complete response; CMR, complete molecular response; MMR, major molecular response; Ph+, Philadelphia-chromosome positive. |
|
|
Response, % (unless otherwise stated) |
Patients with Ph+ ALL (n = 68) |
|---|---|
|
CR† |
100 |
|
CCyR‡ |
100 |
|
Early death |
0 |
|
Molecular response |
|
|
MMR |
98 |
|
After induction |
68 |
|
CMR |
86 |
Safety
The most common Grade ≥3 AEs reported in >15% of patients were infections (93%), increased ALT and AST (31%), hypertension (17%), increased amylase and lipase (16%), pancreatitis (15%), and increased bilirubin (15%).
Presenter’s conclusion
Hyper CVAD plus ponatinib proved highly effective as a frontline combination in patients with Ph+ ALL. This regimen was deemed safe in this patient subset, even more so with risk-adapted dosing of ponatinib. Current investigations into chemotherapy-free options for this patient population are ongoing.
Olverembatinib in adult patients with Ph/BCR-ABL1+ ALL with T315I mutation and relapsed disease7
The first data on olverembatinib in adult patients with Ph/BCR BCR-ABL1+, who either harbored the T315I mutation or experienced disease progression, within China was presented by Weiyang Liu. The study endpoints were efficacy (CR, MRD negativity by MFC and CMR) and safety.
Results
Efficacy
In total, 10 patients were treated. Five patients received ≥2 or more prior TKI’s, two of whom relapsed following ponatinib. With a median follow-up of 3 months, ORR was achieved in 70% of the patients, with an MRD and CMR rate of 71.4% and 57.1%, respectively.
- Of the six patients with a detected T315l mutation still in CR1, 66.7% achieved CMR
- The remaining four patients who were in hematologic relapse achieved a 75% CR rate; one of the two patients with T315L mutation did not respond and the other achieved MRD-negativity
- Olverembatinib was effective in patients who were previously treated with ponatinib
Safety
The incidence of common AEs (cytopenia, elevated transaminases, hypertension, and cardiovascular events) related to third-generation TKIs less frequently occurred in olverembatinib compared with ponatinib. Grade 1 skin pigmentation was observed in four patients, with no drug-related deaths or discontinuations reported.
Presenter’s conclusion
Overall olverembatinib shown encouraging efficacy and safety in adult patients with Ph/BCR BCR-ABL1+ with T315l mutations or relapsed disease.
Ponatinib plus blinatumomab for patients with R/R Ph+ ALL or CML: a phase II subgroup analysis8
Results from the phase II substudy analysis of ponatinib plus blinatumomab in patients with R/R Ph+ ALL and CML in lymphoid blast phase (CML-BP) (NCT03263572) was presented by Macaron.
Results
Efficacy
In total, 20 patients were treated the R/R Ph+ ALL cohort (n = 14) and CML-BP cohort (n = 6). The response and survival rates for the R/R and CML-LBP cohort are summarized in Table 3.
Table 3. Response rates*
|
ALL, acute lymphoblastic leukemia; CML-LBP, chronic myeloid leukemia-lymphoid blast phase; CMR, complete molecular response; CR, complete response; CRi, complete response with incomplete hematologic recovery; EFS, event-free survival; MMR, major molecular response; ORR, overall response rate; OS, overall survival; Ph+, Philadelphia-chromosome positive. |
||
|
Response, % (unless otherwise |
R/R Ph+ ALL |
CML-LBP |
|---|---|---|
|
Hematologic responses |
|
|
|
ORR |
92 |
83 |
|
CR† |
85 |
67 |
|
CRi |
8 |
17 |
|
CMR |
79 |
33 |
|
Survival analysis |
|
|
|
2-year EFS |
46 |
33 |
|
2-year OS |
59 |
60 |
At a median follow-up of 22 months in the R/R cohort, six patients underwent HSCT out of 13 responders and four patients relapsed; there was one death in CR and two patients are in ongoing remission. Moreover, for the CML-BP cohort, three patients experienced relapsed disease and subsequently died out of 5 responders, with two patients still in ongoing remission.
Presenter’s conclusion
Ponatinib and blinatumomab showed promising efficacy and safety in patients with both R/R Ph+ ALL and CML-BP. This combination regimen achieved high rates of deep molecular responses in patients with R/R Ph+ ALL. Conversely, the lower rates of molecular responses seen in patients with CML-BP suggest that alternative approaches should be considered for this subgroup of patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


