All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Novel treatment strategies for adult patients with ALL: Key updates from ASH 2023
Although acute lymphoblastic leukemia (ALL) is the most common neoplasm in children, with survival rates of 80–90%, the landscape is different for adult patients.1 ALL is rare in adults and survival outcomes are unfavorable in comparison with pediatric ALL. With the advancement of therapies, such as T-cell engagers and immunotherapies, results are progressively improving for this cohort of patients for both Philadelphia chromosome-negative (Ph−) and Philadelphia chromosome-positive (Ph+) ALL.1
Here, we summarize five key studies discussing novel treatment approaches for adult patients with ALL presented at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition.
Sequential chemotherapy and blinatumomab: GIMEMA LAL23171
Chiaretti presented results from GIMEMA LAL2317 (NCT03367299), a phase II trial investigating sequential chemotherapy and blinatumomab for improving the rate of early minimal residual disease (MRD) negativity in adult patients with Ph− CD19+ B-cell ALL. The primary objective was the rate of early MRD negativity at the end of Week 14 (Timepoint 3 [TP3]). Median follow-up was 38.1 months, Timepoint 2 (TP2) was after the 1st cycle of consolidation, and timepoint 3 (TP3) was after the 1st cycle of blinatumomab.
- Complete response (CR) was 88%
- MRD-negativity was achieved in, 70% and 72% after TP2 for the whole cohort and paired samples, respectively
- MRD negativity was 93% after TP3, meeting the primary objective; there were no significant differences according to age, but a significant difference according to risk group was reported (p < 0.001)
- The 3-year overall survival (OS) rate was 71%, which differed significantly between age groups (77%, 72%, and 51% for patients aged 18–40, 40–55, and >55 years, respectively)
- The 3-year disease-free survival (DFS) rate was 65%; a significant difference was observed for MRD-negative vs positive patients at TP2 (79% vs 39%, respectively)
- The rate of MRD negativity increased from TP2 to TP3 (70% to 93%, respectively)
In total, 98 patients experienced Grade ≥3 adverse events (AEs), of which 33 were blinatumomab related and 32 were pegylated asparaginase related (Table 1).
Table 1. Most commonly reported Grade ≥3 AEs related to blinatumomab treatment.*
|
Adverse event, % |
|
|
CNS |
67 |
|
Hematologic |
23 |
|
Hepatic |
5 |
|
CRS |
2 |
|
Hypogammaglobulinemia |
2 |
|
AE, adverse event; CRS, cytokine release syndrome; CNS, central nervous system. |
|
Presenter’s conclusions
The primary objective was reached, with 93% of patients experiencing MRD negativity at TP3 and 77% of MRD-positive cases at TP2 achieving MRD negativity after blinatumomab. Blinatumomab was effective across all age groups, with a significant survival improvement in patients aged 40–55 years, and relapses were improved in comparison with historical controls.
Frontline consolidation with nelarabine: GRAALL-2014 vs T ATRIALL trials2
Boissel presented results from the T ATRIALL trial investigating the impact of nelarabine in patients with newly diagnosed T-cell ALL (T-ALL) from the GRAALL-2014 study (2014-002146-44), including 325 participants in total; 112 patients had high-risk features, were in continuous complete remission from the ATRIALL substudy, were assigned to nelarabine plus chlorpheniramine and etoposide, and results were compared with a control cohort (n = 33). The baseline characteristics were comparable between the two cohorts.
- Undetectable MRD at the end of consolidation phase 2 response after the second consolidation was significantly higher with nelarabine treatment than without nelarabine treatment (78% vs 58%, respectively)
- Nelarabine treatment significantly prolonged MRD response compared with no previous nelarabine treatment (65% vs 31%, respectively)
- However, this did not translate to a significant reduction in 3-year cumulative incidence of relapse (CIR) risk (27% vs 47%, respectively) or 3-year DFS (67% vs 49%, respectively)
- There was a significant reduction in the 3-year CIR censored at allogeneic hematopoietic stem cell transplantation (allo-HSCT) for patients treated with vs without nelarabine (29% vs 65%, respectively; p = 0.045)
- There was a benefit in CIR/DFS in patients with non-early T-cell precursor T-ALL and those who were not transplanted
The safety profile was considered manageable, with 37 patients experiencing Grade ≥3 serious AEs and no association of occurrence of AEs across treatment stages (Figure 1).
Figure 1. Serious AEs in all patients included in the substudy according to treatment phase*†
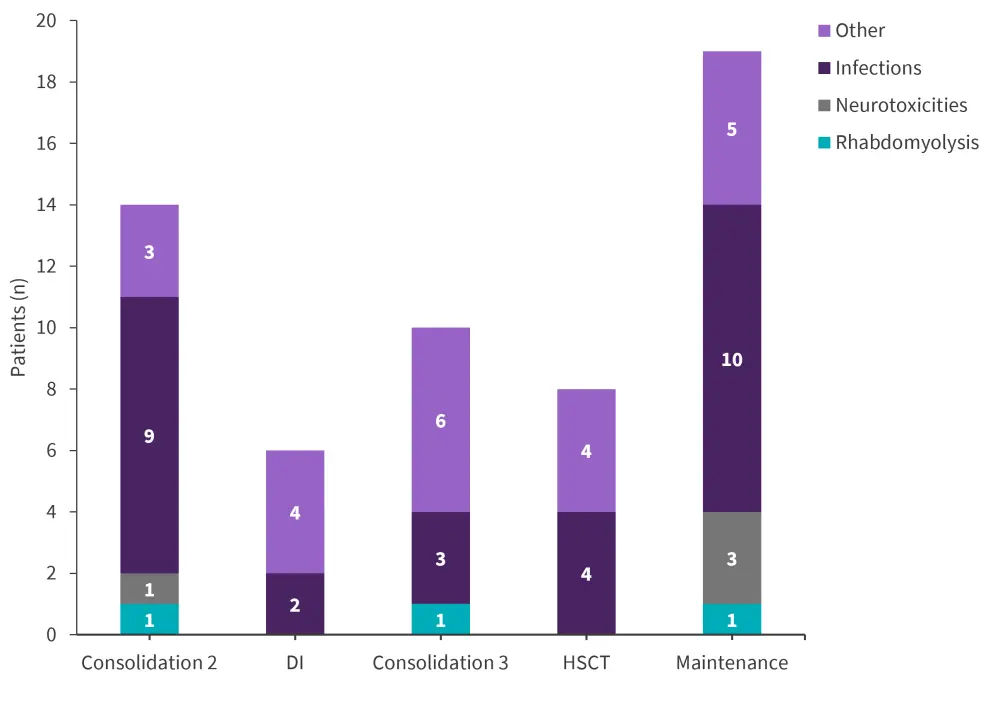
AE, adverse event; HSCT, hematopoietic stem cell transplantation; DI, delayed intensification.
*Adapted from Boissel.2
†N = 120.
Presenter’s conclusions
Nelarabine was well tolerated in adult patients with high-risk T-ALL and MRD was improved compared with standard chemotherapy. Further studies are warranted to reveal the specific patient subgroups that might benefit from nelarabine treatment.
Venetoclax plus blinatumomab: GMALL-Bliven trial3
This phase I multicenter trial (NCT02881086) investigated venetoclax and blinatumomab in combination for adults with relapsed/refractory (R/R) or MRD-positive Ph− B-precursor ALL. The primary endpoint was determination of the maximum tolerated dose ahead of the phase II expansion trial, and the secondary endpoint was efficacy.
- The primary endpoint was not reached
- CR was achieved by 66% (n = 9), 56% achieved molecular CR, and 11% achieved CR with MRD intermediate range
- After a median follow-up of 6 months, 30 and 60-day mortality was 0%
- Cumulative incidence of death in 1 year totaled 22%
Venetoclax in combination with blinatumomab was well tolerated, with five serious adverse events reported (Figure 2). No treatment-related mortality and no dose-limiting toxicities were reported.
Figure 2. Serious adverse events reported in patients with R/R or MRD-positive Ph− precursor B-cell ALL treated with venetoclax plus blinatumomab*
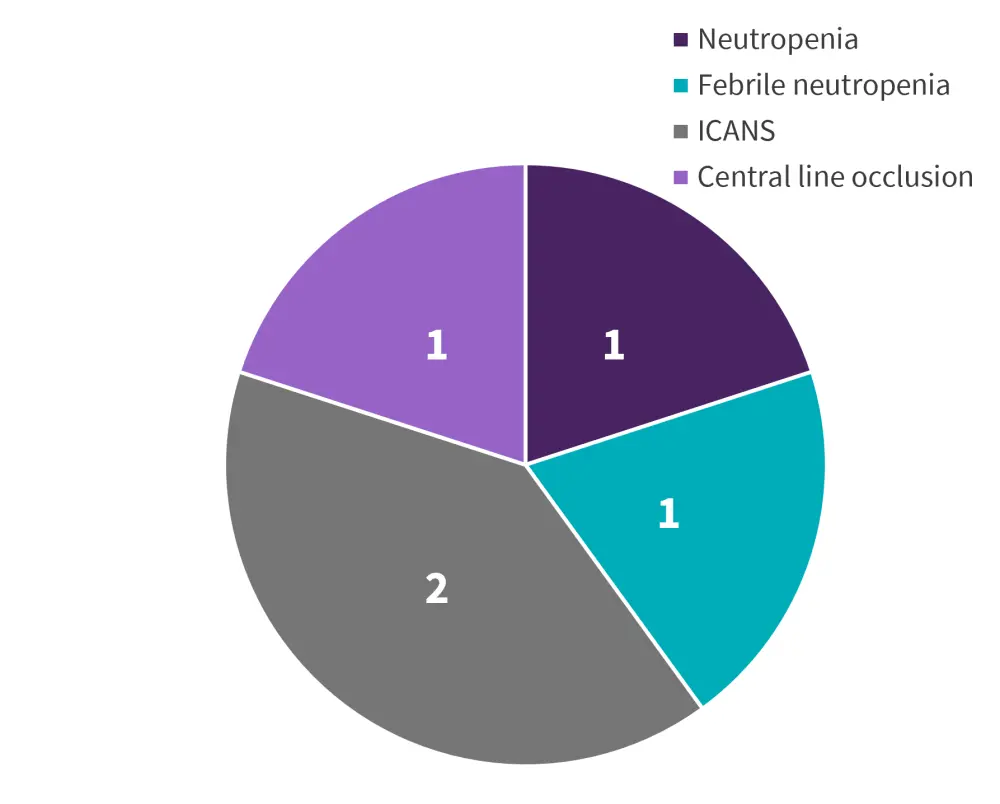
ALL, acute lymphoblastic leukemia; ICANS, immune effector cell-associated neurotoxicity syndrome; MRD, minimal residual disease; Ph−, Philadelphia-chromosome negative; R/R, relapsed/refractory.
*Adapted from Fransecky.3
Presenter’s conclusions
Venetoclax plus blinatumomab was well tolerated in patients with R/R or MRD-positive Ph− precursor B-cell ALL and the maximum tolerated dose was not reached. This treatment showed encouraging efficacy, with 56% achieving molecular CR and the recommended phase II dose of 800 mg once daily. Despite limited patient numbers, the expansion trial currently ongoing in 18 centers will provide further understanding of these results.
BLISSPHALL4
BLISSPHALL (NCT04329325) is a multicenter, phase II study investigating dasatinib plus corticosteroid induction followed by a tyrosine kinase inhibitor (dasatinib [n = 13]; bosutinib [n = 2]; ponatinib [n = 2]) plus blinatumomab consolidation and maintenance therapy in adults with newly diagnosed Ph+ ALL. The primary objective was complete molecular response (CMR) by the end of consolidation (N = 17). Secondary objectives included the safety of blinatumomab + dasatinib, duration of CMR, incidence of relapse, and event-free survival (EFS)/OS.
At a median follow-up of 14.5 months:
- All patients achieved morphologic CR during induction at a median of Day 22
- Three patients achieved CR with incomplete hematologic recovery as an initial response but achieved CR after consolidation
- Overall, 82% achieved MRD negativity by next-generation sequencing
- Ten out of 17 patients achieved CMR and seven did not
- Four patients underwent allo-HSCT in first complete remission and one patient in second complete remission following extramedullary relapse
- Two patients relapsed after achieving CMR and died at 14.5 and 19.3 months after enrollment
AEs were well tolerated, with 42% Grade 3 AEs reported but no Grade 4 or 5 AEs (Table 2).
Table 2. Grade 3 AEs reported in patients with newly diagnosed Ph+ ALL related to treatment with blinatumomab or dasatinib therapy*
|
Most common Grade 3 AE, % |
|
|
Alanine aminotransferase increase |
12 |
|
Aspartate aminotransferase increase |
6 |
|
Nausea |
6 |
|
AE, adverse event. |
|
Presenter’s conclusions
High rates of deep molecular response and low rates of severe toxicities can be achieved in patients with newly diagnosed Ph+ ALL through initial treatment with dasatinib plus corticosteroid induction followed by blinatumomab plus a tyrosine kinase inhibitor. Follow-up studies are required to confirm the durability of responses to blinatumomab consolidation and maintenance in the absence of allo-HSCT.
Ponatinib plus blinatumomab: phase II trial5
Similarly, to the trial summarized by Geyer.4 above, Haddad presents this phase II trial specifically investigating ponatinib plus blinatumomab in adult patients with Ph+ ALL. The primary endpoint was CMR rate (N=62), and the secondary endpoints included EFS, OS, and safety. In total:
- Most patients achieved early CMR with 67% achieving CMR after the first cycle and 84% achieving CMR overall (of which 7 patients were in CMR at the start of therapy)
- CR/CRi was achieved by 98% (of which 22 patients were in CR at the start of therapy).
- So far, one patient received HSCT due to persistent BCR::ABL1 transcripts meanwhile 51 are in ongoing response without HSCT
- After 21 months, 2-year OS and EFS were 90% and 78% respectively and median OS and EFS had not been reached
There were seven relapses in total and four deaths (Table 3).
Table 3. Causes of death in patients enrolled in the phase II trial investigating blinatumomab plus ponatinib*
|
Cause of death, n |
|
|
Early death pre CR |
|
|
Intracranial hemorrhage from prior chemotherapy |
1 |
|
In CR |
|
|
Post-procedural bleeding and hypovolemic shock |
1 |
|
Brain aneurysm |
1 |
|
Post relapse |
1 |
|
CR, complete response. |
|
Presenter’s conclusions
Ponatinib and blinatumomab were shown to have deep and rapid responses with durable remissions; the safety profile was manageable. It was concluded that this combination treatment is a promising, chemotherapy-free, HSCT-sparing regimen for patients with newly diagnosed Ph+ ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


