All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Omission of vincristine/dexamethasone pulse therapy does not adversely affect outcome in low-risk pediatric ALL
Additional pulses of vincristine (V) plus prednisone (Pred) or vincristine plus dexamethasone (Dex) are routinely administered during the maintenance phase of treatment for childhood acute lymphoblastic leukemia (ALL). Early clinical trials performed in the 1970s and 1980s reported improved event-free survival (EFS) with the addition of vincristine + prednisone pulses, and more recent studies have reported superior outcomes with vincristine + dexamethasone compared to vincristine + prednisone.1 However, results from randomized studies of patients with intermediate risk (IR) ALL have been conflicting. While some have reported improved EFS with the addition of vincristine + prednisone or vincristine + dexamethasone, others have observed similar EFS rates regardless of additional pulse therapy.1
Since clinical trial data for patients with intermediate risk ALL are inconclusive, and there is lack of randomized data for low- and high-risk patients, Yang et al.1 evaluated whether additional pulses of dexamethasone + vincristine could be omitted from the second year of maintenance therapy without causing inferior outcomes for all risk groups of pediatric patients with newly diagnosed ALL.
Study design
The study was an open-label, multicenter, randomized, phase III, non-inferiority clinical trial (CCCG-ALL-2015, ChiCTR-IPR-14005706).
Eligible children with newly diagnosed ALL were randomly divided (1:1) into two cohorts: low-risk (LR) and intermediate to high-risk (I/HR), and further sub-divided into control group (receiving additional vincristine + dexamethasone pulses in maintenance therapy) and experimental group (receiving no additional pulses) (Figure 1).
Figure 1. Study design*
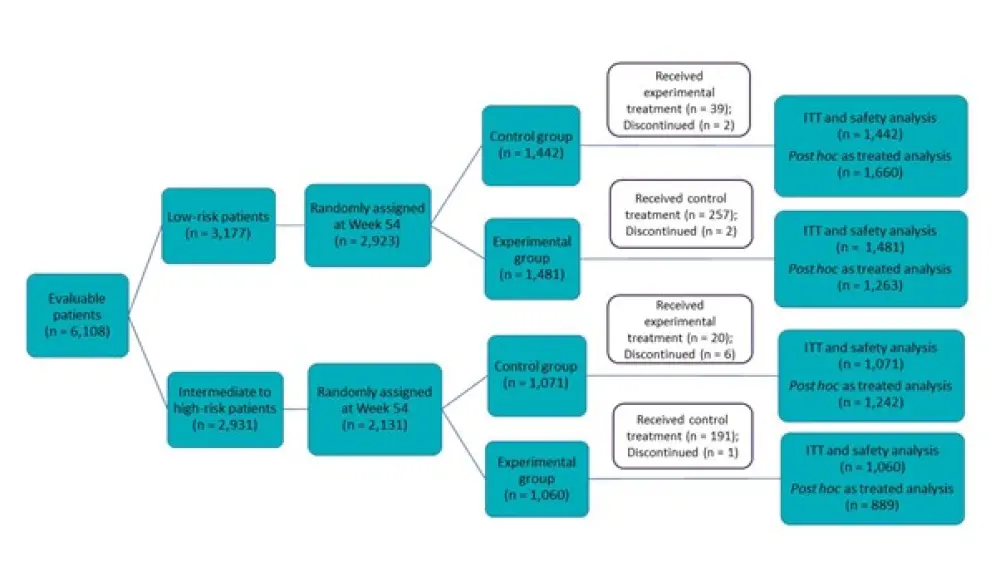
ITT, intention-to-treat population.
*Adapted from Yang et al.2
Treatment plan
The treatment plan for LR and I/HR ALL patients is summarized in Figure 2.
Figure 2. Treatment plan*
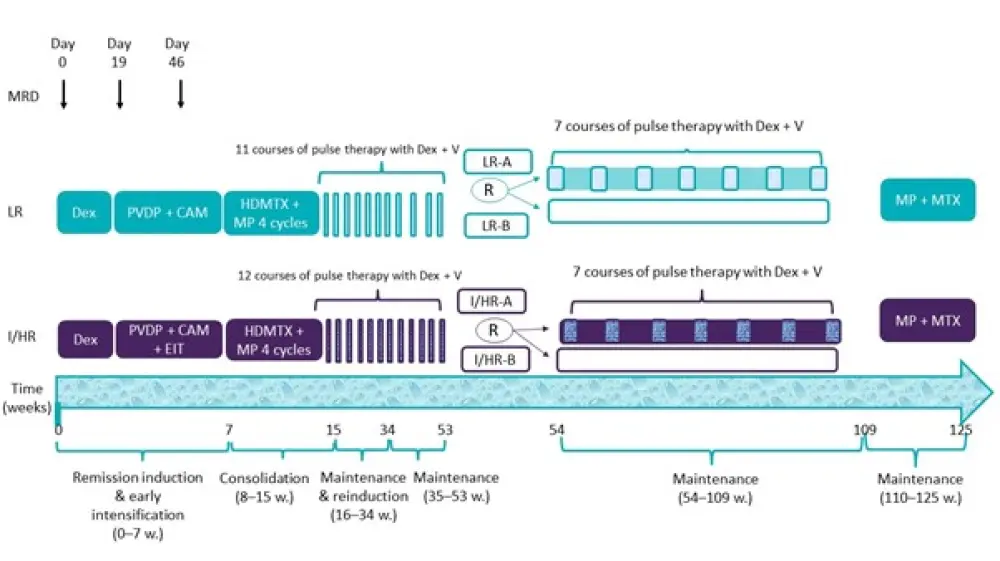
CAM, cyclophosphamide, ara-C, and mercaptopurine; Dex, dexamethasone; EIT, early intensification therapy; HDMTX, high-dose methotrexate; I/HR, intermediate to high risk; I/HR-A, I/HR control group; I/HR-B, I/HR experimental group; LR, low-risk; LR-A, LR control group; LR-B, LR experimental group; MP, mercaptopurine; MRD, minimal residual disease; MTX, methotrexate; PVDP, prednisone, vincristine, daunorubicin, and pegaspargase; R, randomized; V, vincristine; w, weeks.
*Adapted from Yang et al.2
- Maintenance therapy was carried out between weeks 16 and 53, where LR patients received 11 courses of pulse therapy and I/HR patients received 12 courses of pulse therapy with vincristine + dexamethasone, respectively.
- At approximately 1 year of treatment, patients with LR and those with I/HR ALL were separately stratified and randomized to treat with or without seven courses of pulse therapy with vincristine + dexamethasone during continuation treatment between weeks 54 and 109.
- All patients completed treatment with mercaptopurine and methotrexate between weeks 110 and 125.
Study endpoints
- Primary endpoint: Difference in 5-year EFS between the experimental and the control group for both the LR and I/HR cohorts.
- Secondary endpoints: Incidence of central nervous system (CNS) relapse or death during remission, 5-year overall survival (OS) between the experimental and control groups for both the LR and I/HR cohorts.
Post hoc data analysis was also carried out for the IR group to identify the same study endpoints as above.
Results
Patient characteristics
No differences in demographics, disease characteristics, or the levels of minimal residual disease (MRD) of patients at Day 19/46 of remission induction were observed among the groups. However, there was a higher percentage of the KMT2A rearrangement in the experimental group than in the control group (7.1% vs 4.5%) in the I/HR cohort (Table 1).
Table 1. Patient characteristics*
|
C, control; CNS, central nervous system; E, experimental; ETV6-RUNX1 fusion E/R gene; KMT2A, lysine methyltransferase 2A gene; MRD, minimal residual disease; TCF3-PBX1, fusion gene for transcription factor 3 and pre-B cell leukemia transcription factor; WBC, white blood cells. |
||||
|
Characteristic |
Low-risk group |
Intermediate to high-risk group |
||
|---|---|---|---|---|
|
C |
E |
C |
E |
|
|
Age (years), (IQR) |
4.2 (3.0−5.8) |
4.0 (3.0−5.6) |
5.5 (3.2−9.8) |
5.8 (3.1−10) |
|
<1, % |
0 |
0 |
2.9 |
3.5 |
|
1 to <10, % |
98.5 |
98.4 |
72.5 |
71.5 |
|
>10, % |
1.5 |
1.6 |
24.6 |
25.0 |
|
Male, % |
56.7 |
57.3 |
60.0 |
61.5 |
|
WBC <100 (×109 cells/L), % |
99.0 |
98.7 |
80.6 |
80.8 |
|
CNS 1, % |
94.3 |
93.3 |
90.3 |
91.9 |
|
Traumatic lumbar puncture, % |
4.5 |
5.7 |
5.7 |
4.3 |
|
Immunophenotype B, % |
100 |
100 |
80.0 |
80.4 |
|
Ploidy, % |
||||
|
Hyperploidy (>50) |
19.8 |
18.6 |
9.7 |
10.5 |
|
Others |
80.2 |
81.4 |
90.3 |
89.5 |
|
KMT2A rearrangement, positive, % |
0 |
0 |
4.5 |
7.1 |
|
TCF3-PBX1, positive, % |
0 |
0 |
12.4 |
12.3 |
|
ETV6-RUNX1, positive, % |
33.2 |
33.2 |
5.6 |
5.8 |
|
MRD at day 19, % |
||||
|
<0.01 |
59.8 |
60.3 |
34.8 |
35.9 |
|
0.01−0.09 |
20.3 |
19.0 |
9.6 |
8.8 |
|
0.1–0.99 |
20.0 |
20.7 |
16.5 |
17.7 |
|
>1 |
0 |
0 |
39.2 |
37.7 |
|
MRD at day 46, % |
||||
|
<0.01 |
96.8 |
97.1 |
77.2 |
78.2 |
|
0.01–0.09 |
2.3 |
2.2 |
13.7 |
14.4 |
|
0.1–0.99 |
0.9 |
0.7 |
7.7 |
6.5 |
|
>1 |
0 |
0 |
1.5 |
0.9 |
Outcome data
- 5-year EFS was 79.9% (95% CI, 78.7–81.2) and the 5-year OS was 90.3% (95% CI, 89.4–91.2) for all randomized patients.
- No difference in the 5-year EFS and 5-year OS was found between the control and the experimental groups for LR, I/HR or IR cohorts (Table 2).
Table 2. Five-year EFS and OS*
|
C, control; EFS, event-free survival; E, experimental; OS, overall survival. |
||||||
|
Survival |
Low-risk group |
Intermediate to high-risk group |
Intermediate risk group |
|||
|---|---|---|---|---|---|---|
|
C |
E |
C |
E |
C |
E |
|
|
EFS % |
90.3 |
90.2 |
82.8 |
80.8 |
82.7 |
80.7 |
|
p value |
0.90 |
0.90 |
0.90 |
|||
|
OS % |
97.8 |
97.3 |
92.3 |
93.4 |
92.5 |
93.3 |
|
p value |
0.70 |
0.40 |
0.50 |
|||
- With a preset non-inferiority margin of 0.05, the non-inferiority of 5-year EFS and OS was established for the experimental group in the LR cohort (the one-sided 95% upper confidence bound for the difference in the probability of 5-year EFS and 5-year OS were 0.024 and 0.018, respectively).
- On the contrary, borderline inferiority was reported for the experimental group in the I/HR cohort, with a one-sided 95% upper confidence bound for the difference in 5-year EFS probability of 0.055.
- In a post hoc analysis of IR patients alone, the one-sided 95% upper confidence bound for the difference in 5-year EFS probability was also above the preset non-inferiority margin (0.056). In a post hoc analysis by treatment received, there were no significant differences in 5-year EFS and OS for the control and experimental regimen in all cohorts (Table 3).
- Moreover, in the LR, IR, and I/HR cohorts, no differences were observed between the control and the experimental groups in a 5-year cumulative risk of isolated CNS relapse, any CNS relapse, any relapse, and death during remission.
Table 3. Five-year EFS and OS as calculated via a post hoc analysis based on the treatment received*
|
EFS, event-free survival; I/HR, intermediate to high-risk group; IR, intermediate risk group; LR, low-risk group; OS, overall survival. |
||||
|
Risk |
Control, n |
Experimental, n |
p value for 5-year EFS |
p value for 5-year OS |
|---|---|---|---|---|
|
LR |
1,660 |
1,263 |
0.20 |
0.40 |
|
I/HR |
1,242 |
889 |
0.50 |
0.90 |
|
IR |
1226 |
881 |
0.50 |
0.99 |
Adverse events
- In the LR cohort, there were no significant differences in adverse events between the experimental and control groups during the second year of maintenance treatment (Table 4).
- In the I/HR and IR cohorts, Grade 3–4 pneumonia and vincristine-related peripheral neuropathy were higher in the control group than in the experimental group.
- No differences were observed in Grade 5 fatal infection among patients in the control and experimental groups.
Table 4. Adverse events*
|
C, control; E, experimental. |
||||||
|
Grade 3/4 events, % |
Low-risk group |
Intermediate to high-risk group |
Intermediate risk group |
|||
|---|---|---|---|---|---|---|
|
C |
E |
C |
E |
C |
E |
|
|
Infection |
4.3 |
3.4 |
7.0 |
6.0 |
6.7 |
6.0 |
|
Sepsis |
1.0 |
0.7 |
1.8 |
2.0 |
1.7 |
2.0 |
|
Pneumonia |
1.2 |
1.2 |
2.4 |
0.9 |
2.2 |
1.0 |
|
Hyperglycemia |
0.2 |
0.1 |
1.6 |
0.9 |
1.6 |
1.0 |
|
Vincristine-related peripheral neuropathy |
1.0 |
0.7 |
1.6 |
0.6 |
1.6 |
0.6 |
Conclusion
Omission of seven vincristine + dexamethasone pulses in the second year of maintenance treatment did not lead to inferior EFS or OS for patients with low-risk childhood ALL. For patients with intermediate-/high-risk disease, although no significant differences in EFS or OS were observed for patients treated with or without additional pulse therapy, the non-inferiority of the patients who did not receive additional pulse therapy was not demonstrated in this study design. Further studies are therefore needed to determine whether pulse therapy could be omitted without adverse effect for patients with intermediate-risk or high-risk ALL.
Study limitations included the lack of data on treatment doses, prohibiting the evaluation of dose intensity on treatment outcomes, and insufficient resources to capture data for adverse events of Grades 1 and 2. It could, however, be expected that omission of pulse therapy may improve patient quality of life due to fewer treatment-related side effects.
Extended studies are required to determine whether omission of pulse therapy would reduce the long-term effects of treatment with vincristine and glucocorticoids.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

