All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Ph-positive ALL: an overview of the genomic landscape, epidemiology, diagnosis, and treatment strategies
Do you know... Which of the following is the hallmark fusion gene of Philadelphia-chromosome positive acute lymphoblastic leukemia?
Philadelphia-chromosome positive acute lymphoblastic leukemia (Ph+ ALL) is an aggressive subtype of ALL clinically characterized by the presence of the fusion BCR:ABL1 gene. Ph+ ALL is rare in children, with a higher incidence in adults which increases with age.1
In this article, we provide an overview of the genomic landscape, epidemiology, diagnosis, and treatment strategies of Ph+ ALL.
The epidemiology of Ph-like ALL is shown in Figure 1.
Figure 1. Epidemiology of Ph+ ALL infographic*
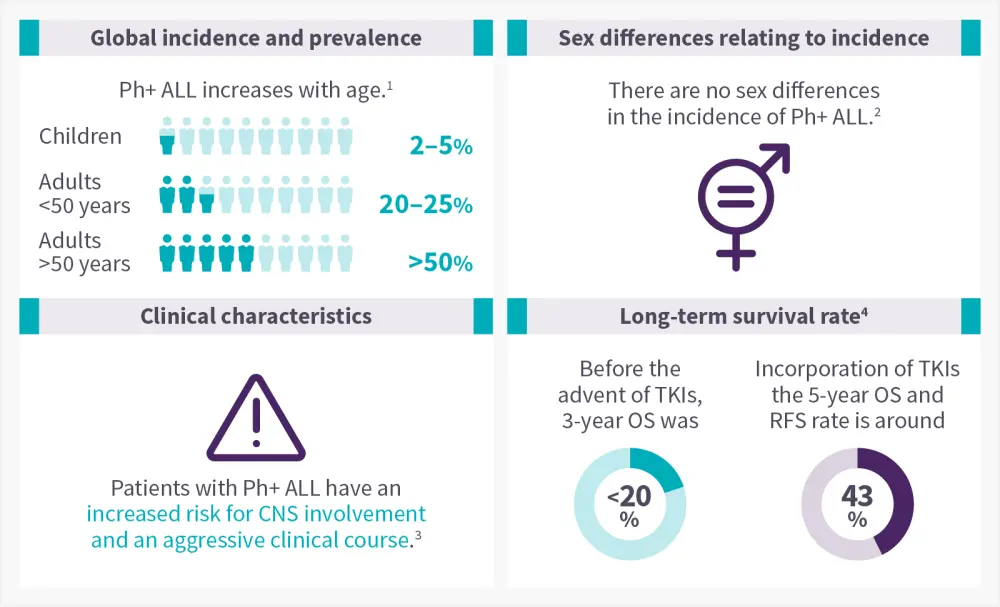
CNS, central nervous system; OS, overall survival; Ph+ ALL, philadelphia chromosome positive acute lymphoblastic leukemia; RFS, relapse free survival; TKI, tyrosine kinase inhibitors.
Data from Foà R and Chiaretti.1; Enrico A and Milone J.2; Dumlao, et al.3; Shi, et al.4
Genomic landscape
The Philadelphia chromosome arises from a reciprocal translocation between the ABL1 oncogene on the long arm of chromosome 9 and a breakpoint cluster region on the long arm of chromosome 22 t(9:22) to form the hallmark BCR:ABL1 fusion gene, as depicted in Figure 2.3,5 This gene encodes the oncogenic protein p190BCR-ABL, which is generated in most cases of Ph+ ALL, or the p210BCR-ABL transcript, which is predominantly found in chronic myeloid leukemia.5,6 Due to the loss of the N-terminus on the ABL1 gene, both transcripts lead to constitutively active tyrosine kinases that alter signaling pathways involved in cell proliferation, survival, and self-renewal, which all play a role in pathogenesis.6,7
Figure 2. Formation of the Philadelphia chromosome*
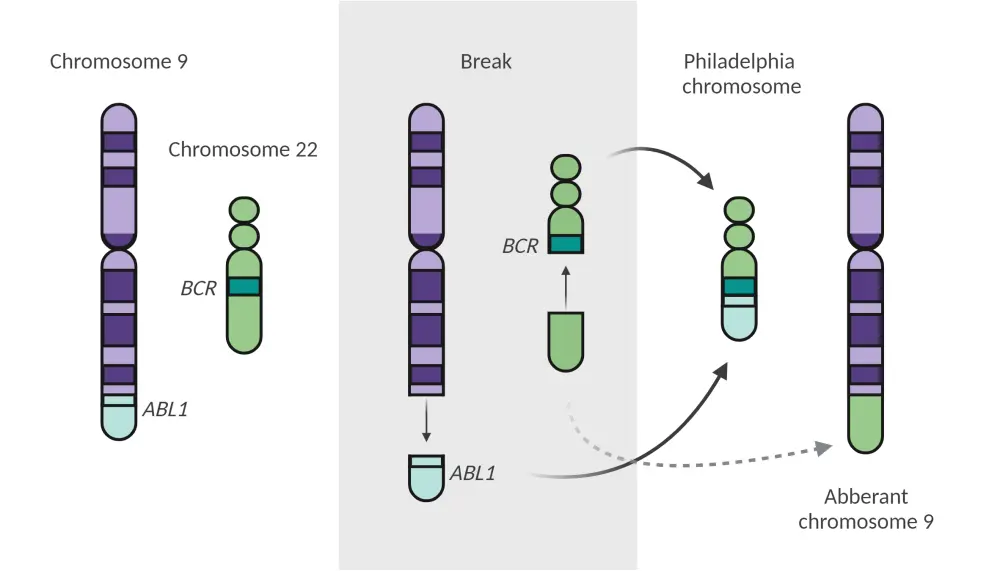
*Adapted from Foà and Chiaretti.1 Created with BioRender.com
As a consequence of hyperactive BCR-ABL1 kinase activity, multiple downstream signaling pathways are altered, including PI3K, AKT, MTOR, RAS, EGFR, MAP-kinase, JNK/SAPK, JAK1–3, the SRC-family kinases LYN, HCK, and FGR, PTPN11, NF-kB, phospholipase C, and STAT5 (Figure 3).7
Figure 3. BCR-ABL1 associated signaling pathways*
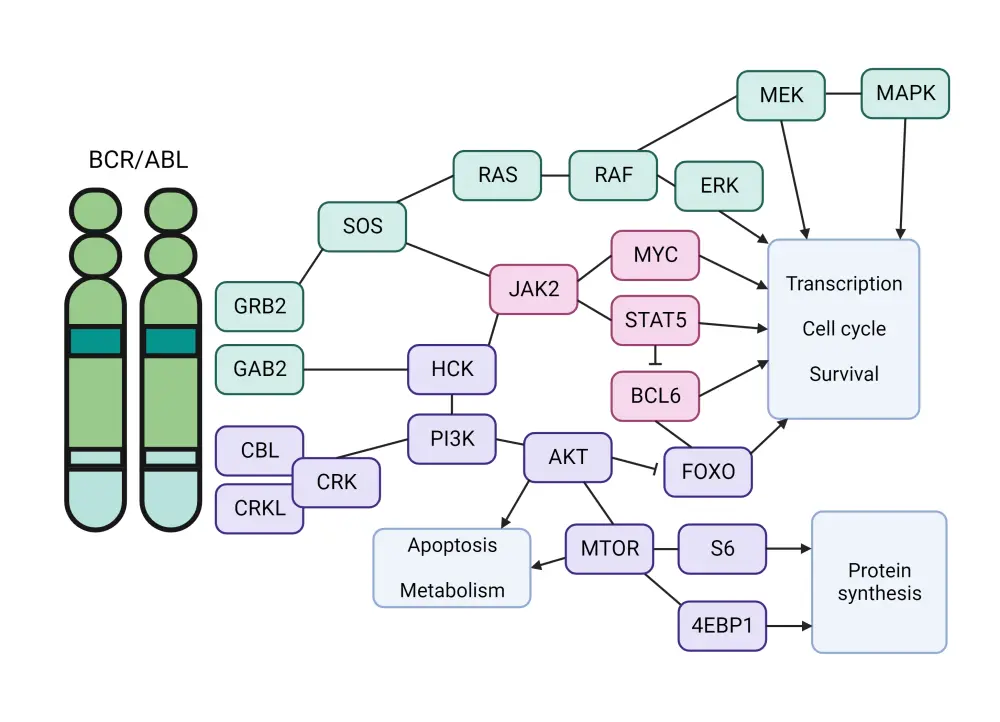
*Adapted from Bernt and Hunger.7 Created with BioRender.com
The most frequent genetic aberrations co-occurring alongside BCR-ABL1 fusions include IKZF1, PAX5, EBF1, and CDKN2A/CDKN2B deletions. IKZF1 mutations occur in 70–80% of Ph+ ALL cases, 90% of which are deletions and 10% are point mutations. Recurring mutations of PAX5 occur in 50% and EBF1 mutations are found in 14% of Ph+ ALL cases; CDKN2A/2B deletions are found more commonly in Ph+ ALL compared to non-Ph+ ALL, at 50% and 30%, respectively. Aside from genetic abnormalities, Ph+ ALL is also characterized by a unique DNA methylation profile, with one recent study detecting 350 differentially methylated regions in Ph+ ALL samples.7
Diagnosis
Rapid and early detection of the BCR:ABL1 rearrangement during the steroid pre-phase is essential in the diagnostic work-up of ALL.8 A bone marrow aspirate should undergo morphological examination to rule out blastic transformation of chronic myeloid leukemia, followed by cytogenetic and molecular examination.5 Cytogenetic analyses by fluorescence in situ hybridization will detect the BCR-ABL gene, while reverse transcriptase polymerase chain reaction (RT-PCR) can more accurately identify BCR-ABL and further define the type of transcript (whether p190 [e2a2] or p210 [b2a2, b3a2]), quantify the levels of BCR/ABL1 rearrangement, and enable adequate minimal residual disease analyses (Figure 4).8
Figure 4. Diagnostic journey of Ph+ ALL*
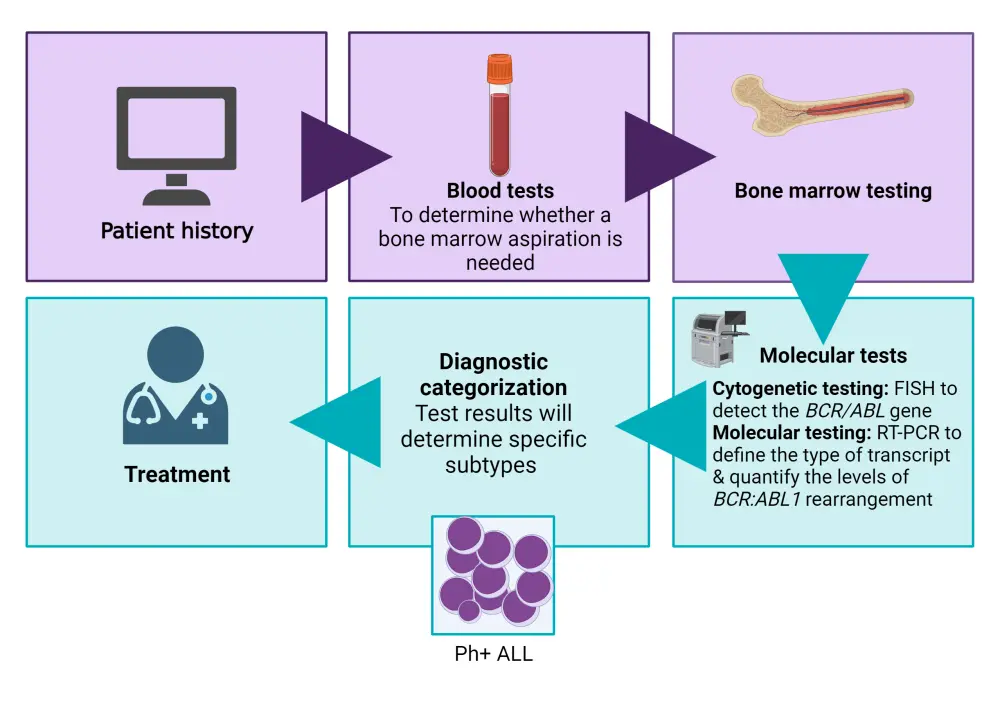
FISH, fluorescence in situ hybridization; Ph+ ALL, Philadelphia chromosome positive acute lymphoblastic leukemia; RT-PCR, reverse transcriptase polymerase chain reaction.
*Data from Leukemia & Lymphoma Society.9 Created with BioRender.com
Management
Historically, Ph+ ALL was associated with very poor prognosis in both adults and children.1 However, incorporation of BCR:ABL1 tyrosine kinase inhibitors (TKIs) and the introduction of monoclonal antibodies, such as the CD19 CD3 bispecific antibody blinatumomab and anti-CD22 antibody inotuzumab ozogamicin (InO), has significantly improved the treatment landscape of Ph+ ALL.10 11 Nonetheless, treatment remains challenging for this disease subtype, particularly in those with relapsed/refractory (R/R) disease.10
For decades, Ph+ ALL treatment relied on induction chemotherapy followed by allogeneic stem-cell transplant (allo-SCT) in first complete remission; however, this strategy yielded suboptimal outcomes (estimated long-term survival rates, 10–35%).11 Trials on frontline and R/R Ph+ ALL are summarized in Figure 5 and Figure 6, respectively.
Figure 5. Summary of frontline trials in Ph+ ALL*
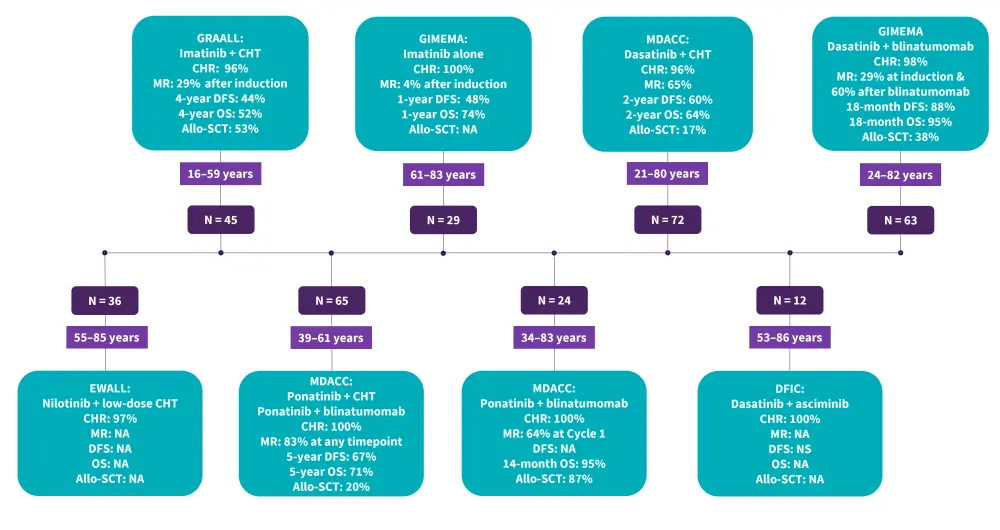
ALL, acute lymphoblastic leukemia; Allo-SCT, allogeneic stem cell transplantation; CHR, complete hematologic remission; CHT, chemotherapy; DFCI, Dana Farber Cancer Institute; DFS, disease-free survival; EWALL, European Working Group for Adult Acute Lymphoblastic; GRAALL, Group for Research on Adult Acute Lymphoblastic Leukemia; MDACC, MD Anderson Cancer Center; MR, molecular response; NA, not available; OS, overall survival; Ph+, Philadelphia chromosome-positive.
*Data from Foà and Chiaretti.
Figure 6. Summary of ongoing and completed trials for R/R Ph+ ALL*
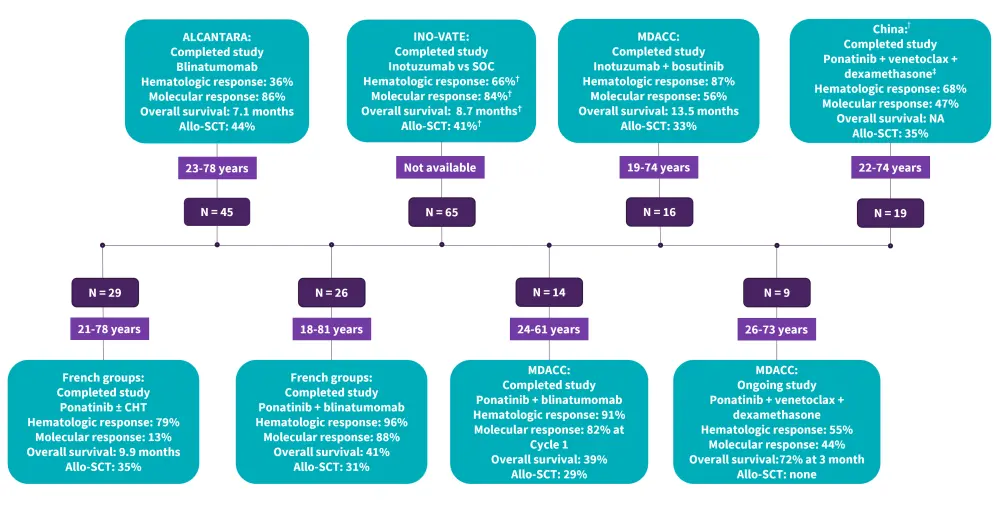
ALL, acute lymphoblastic leukemia; Allo-SCT, allogeneic stem cell transplantation; CHT, chemotherapy; MDACC, MD Anderson Cancer center; Ph+, Philadelphia chromosome-positive; R/R, relapsed and refractory; SOC, standard of care.
*Data from Foà and Chiaretti.1
†This regimen was administered only in patients with T315I mutations.
First-generation TKIs8,11
Imatinib is a first-generation TKI approved for the treatment of newly diagnosed and R/R Ph+ ALL. Clinical trials have revealed that the addition of imatinib to standard chemotherapy has led to significant improvements in CR rates and OS in patients with Ph+ ALL. In the UKALLXII/ECOG2993 trial (NCT00002514), sequential addition of imatinib to intensive chemotherapy during the induction phase resulted in a CR rate of 92% vs 82% in the pre-imatinib cohort, demonstrating this regimen as a viable therapeutic option.
In a randomized trial, addition of imatinib during induction with high-intensity and low-intensity chemotherapy showed similar responses; however, early deaths were high in the high-intensity chemotherapy arm (6.7% vs 0.7%). Imatinib, when combined with hyper-CVAD, achieved an OS rate of 43% and complete molecular remission (CMR) rate of 45%. Long-term follow-up studies showed similar OS among patients with or without allo-SCT, suggesting that transplant may not be necessary in all patients with Ph+ ALL.
Second-generation TKIs11
Dasatinib and nilotinib are second-generation TKIs that are more potent at targeting BCR-ABL1 than imatinib, with clinical trials showing improved responses and survival rates in patients with Ph+ ALL. Both treatments, when combined with different chemotherapy regimens or supportive care, achieved high CR and CMR rates in various populations of patients with Ph+ ALL, including adults, older adults, and children. Some studies highlight pharmacokinetic and clinical differences between the two drugs, such as the higher dose of dasatinib needed in patients at risk of CNS relapse and better outcomes with dasatinib compared with imatinib in pediatric patients. Overall, dasatinib and nilotinib are promising options for patients with Ph+ ALL who may benefit from less intensive chemotherapy or alternative transplantation strategies; however, some patients treated with either dasatinib or nilotinib relapsed due to the presence of T315I mutations.
Third-generation TKIs11
Ponatinib, a third-generation TKI, can effectively target Ph+ ALL harboring the T315I mutation and is an option for second-line treatment; however, toxicity (arterial occlusive events and pancreatitis) remains a concern. Several studies have shown improved response and survival outcomes with ponatinib in patients with ALL when combined with different regimens, such as hyper-CVAD, high-dose chemotherapy, and steroids. However, ponatinib can also cause problems in the arteries and pancreas, which may require lower doses and preventive medications.
Combination with TKIs
Blinatumomab is an anti-CD3/CD19 bispecific antibody which has shown promising results in combination with TKIs or corticosteroids for patients with R/R Ph+ B-ALL. Blinatumomab can induce high rates of CR, CMR, and OS, while reducing the need for allo-SCT and chemotherapy. Inotuzumab ozogamicin, an anti-CD22 antibody-drug conjugate, has also shown significant activity when combined with a third-generation TKI (bosutinib) in patients with R/R Ph+ B-ALL, achieving high CR/CR rates.1,11,12
The role of allo-SCT in Ph+ ALL
The role of allo-SCT for patients with Ph+ ALL in first CR is controversial, with some studies demonstrating long-term survival outcomes with chemotherapy plus TKIs for some patients who do not undergo allo-HCT.3,11 In one frontline study evaluating hyper-CVAD plus TKIs, patients who did not undergo SCT in CR1 and achieved a CMR by Month 3 had a significantly longer median OS when compared to those with a lower response (127 vs 38 months, respectively); this suggests that these patients may obviate the need for allo-SCT.11 However, patients with high-risk features, such as persistent minimal residual disease, IKZF1 plus or ABL1 mutations may benefit from allo-SCT in CR1.1
Key Guidelines and organizations
- ESMO clinical practice guidelines
- NCCN guidelines for physicians
- Cancer Research UK: treatment of acute lymphocytic leukemia.
- American Cancer Society: treatment of acute lymphocytic leukemia.
- Leukemia & Lymphoma Society: treatment of acute lymphocytic leukemia.
- NHS: treatment for acute lymphoblastic leukemia.
- NCCN guidelines for patients
- Patient empowerment network
- CancerCare
- Leukemia research foundation
- Know ALL
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

