All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Phase I results show venetoclax and navitoclax combination is tolerable and associated with responses in pediatric patients with R/R ALL and LL
The prognosis is poor for pediatric patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LL); there is an unmet need for more effective therapeutic approaches to improve outcomes.
The combination of BCL-2 and BCL-XL inhibitors have shown antileukemic activity in ALL mouse models, and the BCL-2/BCL-XL inhibitor navitoclax was investigated in patients with R/R ALL. However, standard-dose navitoclax treatment has been associated with dose-limiting toxicities (DLTs), primarily thrombocytopenia, and the aim of this previous study was related to navitoclax monotherapy only.
The combination of venetoclax, a potent, oral BCL-2 inhibitor, with navitoclax was later investigated in a phase I trial in adult and pediatric patients with ALL and LL (NCT03181126), in order to evaluate whether the addition of venetoclax to low-dose navitoclax would improve efficacy in BCL-2 inhibition without the dose-limiting thrombocytopenia. Results were presented during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition by Jeffrey E. Rubnitz.1 We are pleased to summarize these results.
Study design
- Phase I, multicenter, open-label, dose-escalation study (Figure 1)
- Eligibility criteria were as follows:
- Measurable R/R ALL or radiologically confirmed LL
- Age ≥ 4 to < 18 years
- Body weight ≥ 20 kg
- Adequate organ function and performance status
- Primary endpoints were safety, including rate of DLTs and adverse events (AEs), and pharmacokinetics
- Secondary endpoints included complete response (CR) rate, progression-free survival (PFS), overall survival (OS), and percentage of patients undergoing stem cell transplantation (SCT) or chimeric antigen receptor (CAR) T-cell therapy
- Exploratory endpoints were minimal residual disease (MRD) and biomarkers
Figure 1. Study design1
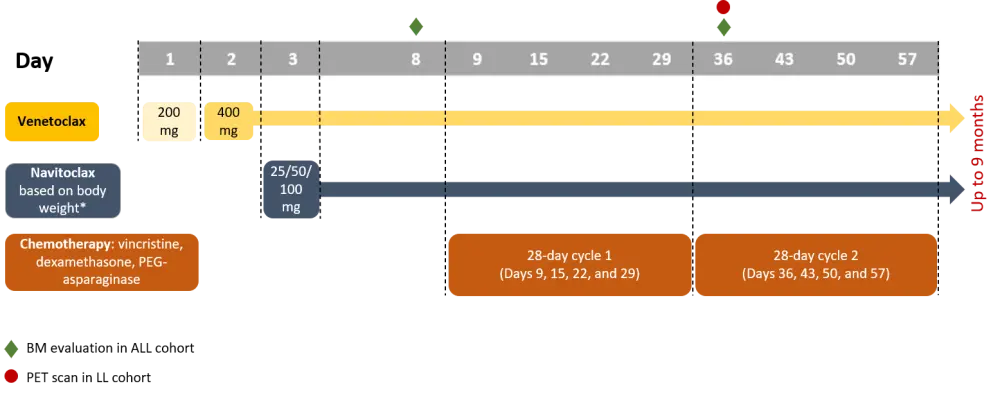
ALL, acute lymphoblastic leukemia; BM, bone marrow; LL, lymphoblastic lymphoma; PEG, pegylated; PET, positron emission tomography.
*Body weight ≥45kg: 25, 50, 100 mg; body weight <45 kg: 25, 50 mg.
- Study protocol was amended to allow starting chemotherapy as early as Day 1
- Patients with ALL underwent bone marrow evaluation on Day 8 (before starting chemotherapy) and on Day 36
- Patients with LL underwent positron emission tomography (PET) scan on Day 36
- A 21-day dosing schedule of 400 mg venetoclax followed by a 7-day off period, plus navitoclax 50 mg (weight ≥ 45kg) or 25 mg (weight < 45 kg), was evaluated in a safety expansion cohort
Patients
- The number patients included was 18 (dose escalation, n = 12; safety expansion, n = 6) with a median age of 10 years (range, 6–16)
- More than half of patients (55%) had a Larnsky or Karnofsky performance status of ≥ 90
- Most patients (72%) had B-cell ALL (Table 1)
Table 1. Pediatric patient characteristics1
|
Characteristic |
N = 18 |
|---|---|
|
ALL, acute lymphoblastic leukemia; B-ALL, B-cell ALL; BM, bone marrow; CAR, chimeric antigen receptor; CI, confidence interval; LL, lymphoblastic lymphoma; SCT, stem cell transplantation; T-ALL, T-cell ALL; T-LL, T-cell LL. |
|
|
Male sex, n (%) |
10 (56) |
|
Primary diagnosis, n (%) |
|
|
Median baseline BM blasts, % (range) |
69.5 (2.0–97.0) |
|
Prior therapies, n |
|
|
Median time since last therapy, months (range) |
1.6 (0–174) |
|
Median time on study, months (95% CI) |
10.4 (3.6–15.2) |
|
Median duration of venetoclax exposure, months (range) |
1.4 (0–4.9) |
|
Median duration of navitoclax exposure, months (range) |
1.4 (0.2–4.8) |
In the dose-escalation cohort (n = 12), six patients received 25 mg, five received 50 mg, and one received 100 mg navitoclax.
Results
Safety
All patients experienced adverse events (AEs) of any grade, and the rate of Grade 3/4 AEs was 89%. Grade 3/4 AEs related to venetoclax or navitoclax occurred in 56% of patients each. DLTs were reported in two patients, including delayed count recovery (25 mg navitoclax) and sepsis (50 mg navitoclax). Table 2 summarizes Grade 3/4 AEs reported in ≥ 15% of patients. There were no Grade 5 treatment-emergent AEs or tumor lysis syndrome reported. Eight patients died due to disease progression.
Table 2. Grade 3/4 AEs1
|
AE, adverse event; ALT, alanine transaminase. |
|
|
AE, % |
Grade 3/4 (≥ 15% of patients) |
|---|---|
|
Any |
89 |
|
Hematologic AEs |
|
|
Febrile neutropenia |
50 |
|
Neutropenia |
33 |
|
Thrombocytopenia |
33 |
|
Anemia |
28 |
|
Leukopenia |
28 |
|
Nonhematologic AEs |
|
|
Hyperglycemia |
17 |
|
ALT increased |
17 |
Efficacy
Efficacy outcomes and response rates amongst different cohorts are summarized in Table 3. The rate of CR/CR with incomplete recovery (CRi)/CR without platelet recovery was 56%.
Table 3. Summary of efficacy analysis1
|
ALL, acute lymphoblastic leukemia; B-ALL, B-cell ALL; CI, confidence interval; CR, complete response; CRi, CR with incomplete marrow recovery; CRp, CR without platelet recovery; DOR, duration of response; LL, lymphoblastic lymphoma; MRD, minimal residual disease; NE, not evaluable; NR, not reached; OS, overall survival; PR, partial response; T-ALL, T-cell ALL. * Among those who achieved CR/CRi/CRp |
||||
|
Outcome |
B-ALL |
T-ALL |
LL |
All patients |
|---|---|---|---|---|
|
CR/CRi/CRp, % |
62 |
33 |
50 |
56 |
|
PR, % |
15 |
0 |
0 |
11 |
|
MRD-negativity, %* |
63 |
100 |
100 |
70 |
|
Median time to first response, months (range) |
1.0 (0.2–2.1) |
1.3 (1.3–1.3) |
1.2 (1.2–1.2) |
1.0 (2.9–NE) |
|
Median DOR, months (95% CI) |
3.5 (0.7–10.4) |
NR |
NR |
3.5 (0.7–10.4) |
|
Median OS, months (95% CI) |
11.4 (3.2–NE) |
2.9 (1.2–NE) |
NR (2.0–NE) |
11.4 (2.9–NE) |
- Eight patients (44%) proceeded to SCT or CAR T-cell therapy, and all patients achieved CR or CRp. Most of these patients were alive at the time of last follow-up
- OS at 1 year in all patients was about 50%
BH3 profiling and genomic analysis
- The dependency of blasts on BCL-2, BCL-XL and MCL-1 was evaluated via BH3 profiling, showing more diversity in the B-ALL patients compared to early T-cell precursor and T-ALL patients
- Patients with BCL-2 or BCL-XL dependency achieved responses; none of the patients showed MCL-1 dependency
- Preliminary genomic analysis demonstrated that there was a variation of low- and high-risk subtypes among patients, and responses were observed in both risk groups
- Amongst BCL2, BCL2L1, and MCL1, baseline gene expression was highest with MCL1, however there was no dependency on MCL1. Although small, BCL2 and BCL2L1 gene expression was slightly higher in responders versus non-responders
Conclusion
The study population included heavily pre-treated pediatric patients with R/R ALL or LL. The combination of venetoclax and navitoclax plus chemotherapy was well tolerated, however, delayed count recovery was still an important safety concern. Response rates were promising amongst patients. BH3 profiling and genomic analysis are currently ongoing. Biomarker results suggests the venetoclax and navitoclax combination may be a valid option to target BCL-2 and BCL-XL.
The recommended phase II dose is 400 mg venetoclax with 25 mg navitoclax for patients weighing < 45 kg, or 50 mg navitoclax for patients weighing ≥ 45 kg, and preparations for this trial are currently ongoing.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

