All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Phase II trial results of inotuzumab ozogamicin in older patients with de novo B-ALL: GMALL-INITIAL1 trial
Acute lymphoblastic leukemia (ALL) represents 5% of all newly diagnosed leukemias in older patients aged 55–70 years. With recent advances mainly being focused on younger populations, older patients have seen limited prognostic benefit. Survival outcomes for elderly patients remain poor with age-adapted, dose-reduced chemotherapy regimens, even after moderately intensive doses; this patient subset is ineligible for unmodified pediatric-based therapies.1
The ALL Hub has previously reported on the efficacy and safety of inotuzumab ozogamicin (InO) as a frontline treatment for older patients (>55 years) with Philadelphia-chromosome-negative (Ph−) ALL.1 InO plus mini-hyperfractionated cyclophosphamide, vincristine, and dexamethasone has demonstrated promising survival rates in older patients with Ph− ALL.1,2
Below, we summarize recent efficacy and safety data from the phase II GMALL-INITIAL-1 trial (NCT03460522) investigating InO induction in patients with de novo Ph− ALL, presented by Stelljes at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition in 2022.2
Study design
This study was conducted across 13 sites in Germany and included patients enrolled between June 2018 and April 2021. Eligible patients were aged >55 years, had CD22+ Ph− B-ALL with or without CNS involvement, and had received no previous ALL-specific treatment (except prephase). The treatment schedule consisted of three cycles of InO induction, conventional chemotherapy consolidation, and subsequent maintenance (Figure 1).
Figure 1. Study design*
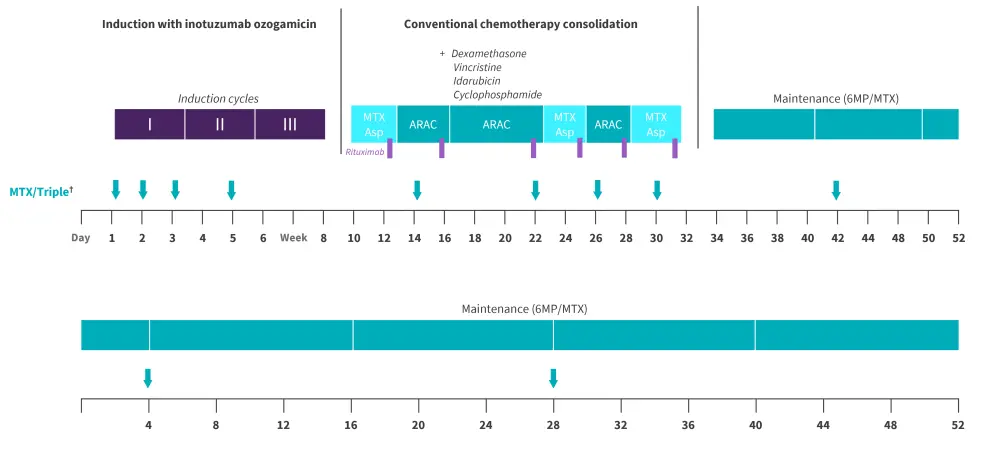
ARAC, cytarabine; Asp, asparaginase; 6MP, 6-mercaptopurine; MTX, methotrexate.
†Administered intrathecally.
*Adapted from Stelljes, et al.2
The primary endpoint was event-free survival at the 12-month follow-up. An event was defined as any of the following: persisting bone marrow blasts after two cycles of InO, relapse, or death. An event rate of <40% at 12 months was considered promising for further evaluation.
Secondary endpoints included remission rates, rate of minimal residual disease negativity, remission after induction treatment, relapse-free survival and overall survival at two 2-years, rate of deaths during induction/and or death in complete remission (CR), and the proportion of patients with molecular relapse.
Results
Updated efficacy2
Of the 45 patients included, 43 were evaluable for hematological remission and follow-up assessments. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
B-ALL, B-cell acute lymphoblastic leukemia. |
|
|
Characteristic, n (unless otherwise stated) |
n = 43 |
|---|---|
|
Median age (range), years |
64 (56–80) |
|
>65 years |
20 |
|
>70 years |
12 |
|
Sex |
|
|
Male |
20 |
|
Female |
23 |
|
Common ALL features |
38 |
|
Pro B-ALL features |
5 |
|
Blasts >20% positive for CD20 |
17 |
|
Median CD22 expression (range) |
69 (21–99) |
|
Blasts >20-40% positive for CD22 |
5 |
|
Blasts >40-60% positive for CD22 |
10 |
|
Blasts >60% positive for CD22 |
28 |
Overall, induction with InO resulted in high remission rates (Table 2).
Table 2. Response rates*
|
CR, complete remission; CRi, complete remission with incomplete count recovery; InO, inotuzumab ozogamicin; MRD, minimal residual disease. |
|
|
Hematological remission, % (unless otherwise stated) |
n = 43 |
|---|---|
|
CR/CRi after 2 induction cycles |
100 |
|
Patients receiving three cycles of InO |
94† |
|
Early deaths within the first 6 months |
0 |
|
Evaluable for MRD |
|
|
MRD negative after 2nd induction cycle |
53 |
|
MRD negative after 3rd induction cycle |
74‡ |
|
Median time between 1st and 2nd induction (range), days |
21 (21–31) |
|
Median time between 2nd and 3rd induction (range), days |
28 (27–33) |
|
Median time and between 3rd induction 1st consolidation (range), days |
30 (26–42) |
- Within the first year, a total of five events occurred; two patients with relapsed disease and three deaths in CR
- The primary endpoint was met, with an event rate of 12%
- At a median follow-up of 697 days, the study showed promising overall survival and event-free survival rates at 1 and 2 years (Figure 2)
Figure 2. 1 and 2-year OS and EFS*
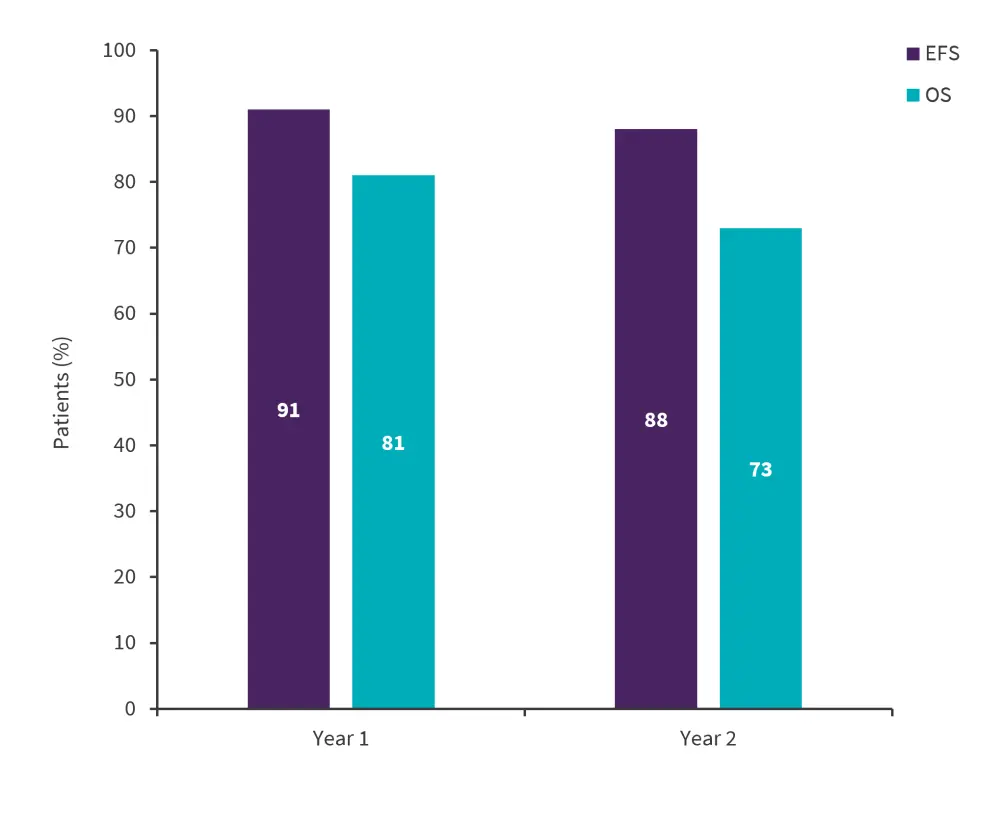
EFS, event free survival; OS, overall survival.
*Adapted from Stelljes, et al.1
As of July 2022, subsequent treatment given includes blinatumomab in seven patients (five for relapse and two for minimal residual disease), InO in three patients (for relapse), CAR-T-cell therapy in one patient (for relapse), and allogeneic hematopoietic cell transplantation in ten patients (six after relapse and four in 1st CR).
Updated safety2
Adverse events mostly occurred in the first induction cycle of InO and were likely to be disease related. The three most common adverse events overall were leukocytopenia, anemia, and thrombocytopenia (Table 3).
Table 3. Grade 3–4 AEs reported after INO induction*
|
AE, adverse event; CTC, Common Terminology Criteria; GOT; glutamic oxalacetic transaminase; GPT; glutamic pyruvic transaminase; INO, inotuzumab ozogamicin; VOD, veno-occlusive disease. †One patient reported with suspected VOD after 2nd induction cycle. |
||||
|
CTC AEs > 5% (3-4; 4.0) |
Induction 1 |
Induction 2 |
Induction 3† |
|
|---|---|---|---|---|
|
Leukocytopenia |
74 |
19 |
2 |
|
|
Anemia |
37 |
5 |
0 |
|
|
Thrombocytopenia |
49 |
7 |
2 |
|
|
Elevation of GOT/GPT |
14 |
0 |
0 |
|
|
Elevation of bilirubin |
2 |
0 |
0 |
|
|
Hyperglycemia |
12 |
5 |
2 |
|
|
Febrile neutropenia |
5 |
0 |
0 |
|
|
VOD |
0 |
2 |
0 |
|
Conclusion
In this study, InO as induction therapy demonstrated high efficacy and a manageable toxicity profile in older patients with B-ALL. The study reported high remission rates, promising survival outcomes and no early deaths in the first 6 months. These results provide a basis for the integration of InO induction in future treatment recommendations; however, evaluation in prospective randomized trials is needed.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


