All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Progress of targeted therapies and immunotherapies in T-ALL/LBL
Do you know... What is the molecular target of ruxolitinib, a therapeutic strategy being investigated for patients with T-cell acute lymphoblastic leukemia?
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignant neoplasm accounting for 10–15% of pediatric and 20–25% of adult cases.1 Despite the significant improvements in treatment outcomes, the prognosis for adult patients with relapsed/refractory (R/R) T-ALL, particularly those aged >60 years, remains poor. As such, there is an urgent need for new therapeutic strategies. A better understanding of the genetic landscape has allowed the identification of a range of new therapeutic targets for T-ALL.1
The ALL Hub has previously reported on the treatment landscape of T-ALL. Here, we summarize the key points from a review published by Huang et al.1 in Annals of Hematology on the progress and challenges of targeted therapies and immunotherapies for T-ALL.
Molecular targeted therapies
Comprehensive cytogenetic testing and molecular analyses have identified numerous genetic biomarkers and therapeutic targets for T-ALL. The pre-clinical and clinical efficacy for some of these targets are summarized in Table 1.
Table 1. Pre-clinical/clinical efficacy of molecular targeted therapies in T-ALL*
|
CAR-T, chimeric antigen receptor T-cell; CR, complete remission; ETP-ALL, early T-cell precursor lymphoblastic leukemia; MRD, minimal residual disease; T-ALL, T-cell acute lymphoblastic leukemia; NR, not reported; OS, overall survival; PFS, progression-free survival. |
|||
|
Treatment |
Targets |
Clinical model/cell types |
Clinical efficacy |
|---|---|---|---|
|
Preclinical models |
|||
|
BMS-906024 |
NOTCH1 |
Ex vivo/Primary T-ALL activated cells |
NOTCH target genes downregulation |
|
Ruxolitinib |
JAK/STAT pathway |
In vitro/in vivo |
Decrease in leukemia burden; anti-proliferative effects; survival prolongation |
|
Tofacitinib + Dexamethasone |
JAK/STAT pathway |
Ex vivo/in vivo/primary T-ALL cells |
Synergistic cytotoxicity and apoptosis; leukemia burden decrease |
|
Dasatinib |
ABL1/SRC |
Ex vivo/in vitro/primary T-ALL cells |
Anti-proliferative effects |
|
Chidamide |
HDAC |
In vitro/leukemia cell lines |
Anti-proliferative effects; apoptosis; cell cycle arrest |
|
ARV-825/JQ1 |
BRD4 |
Ex vivo/in vivo/in vitro/primary T-ALL cell lines |
Anti-proliferative effects; c-Myc down-regulation; leukemia burden decrease |
|
Venetoclax + SP2509 |
BCL2 + LSD1 |
In vitro/ex vivo/in vivo/T-ALL cell lines |
Synergistic anti-proliferative effects and apoptosis in ETP-ALL cells; leukemia burden decrease |
|
Clinical models |
|||
|
Treatment |
Targets |
No. of enrolled patients (N) |
Efficacy outcomes |
|
Navitoclax + venetoclax + chemotherapy |
BCL2 |
19 |
CR: 52.6% MRD-CR: 31.6% 1-year OS: 66.7% |
|
Isatuximab |
CD38 |
14 |
NR |
|
CD7-directed CAR-T |
CD7 |
20 |
CR: 85% 2-year PFS: 36.8% 2-year OS: 42.3% |
|
Pembrolizumab |
PD-1 |
12 |
NR |
NOTCH1 signaling pathway
Activation of NOTCH1 signaling occurs in >70% of T-ALL cases. Gamma secretase inhibitors that block the cleavage and activation of intracellular NOTCH1 fragments have been investigated in patients with T-ALL. One patient treated with the gamma secretase inhibitor, BMS906024, achieved a complete hematologic response with a deep molecular response after two cycles, and one patient with R/R T-ALL treated with the NOTCH inhibitor CB-103 achieved a complete response (CR) within 1 week and progressed to allogeneic stem cell transplantation (allo-HSCT). A phase I/II multicenter trial is currently investigating CB-103 in adult patients with advanced hematological malignancies (NCT03422679).
A multicenter phase I study evaluating NOTCH inhibitor crenigacestat in 31 patients with T-ALL and five patients with T-cell lymphoblastic lymphoma (T-LBL), showed a median event-free survival of 1.18 months. The study demonstrated the limited clinical activity of this target in patients with R/R T-ALL/LBL.
JAK/STAT pathway
Ruxolitinib, a JAK1 and JAK2 inhibitor, has shown a reduction in blast count in blood and spleen in early T-cell precursor lymphoblastic leukemia (ETP-ALL) xenograft models and prolonged survival in a patient with T-ALL patient with cutaneous relapse. Tofacitinib is a JAK3 inhibitor that has demonstrated synergistic effects with prednisolone and dexamethasone both in vitro and in vivo. A phase I/II study is currently assessing the efficacy of ruxolitinib plus L-asparaginase, vincristine, and prednisone in adult patients with R/R ETP-ALL (NCT03613428).
Targeted kinase inhibitors
NUP214::ABL1 is the most common ABL1 rearrangement in T-ALL. The dual ABL1/SRC kinase inhibitor, dasatinib, has shown a complete hematologic and cytogenetic remission after 3 weeks and was well-tolerated with no side effects in a patient with NUP214::ABL1-positive T-ALL. Another SRC kinase family that is highly expressed in T-ALL is the lymphocyte-specific kinase which could be a suitable therapeutic target in T-ALL.
Given the low BCL2-acitivity and venetoclax resistance exhibited in dasatinib-sensitive primary T-ALL cells, the combination of dasatinib plus venetoclax could be an effective treatment strategy in patients with T-ALL. A phase III study is currently evaluating imatinib combined with chemotherapy in newly diagnosed patients with Philadelphia chromosome positive ALL (NCT03007147).
Target therapies for epigenetic changes
The histone deacetylase (HDAC) inhibitor, chidamide, has demonstrated higher overall response rates and better progression-free survival compared with historical chemotherapy in patients with R/R T-ALL. A prospective study is currently evaluating the efficacy and safety of chidamide combined with the PDT-ALL-2016 protocol in patients with T-ALL (NCT03564704). ARV-825, a BRD4 inhibitor has shown inhibition cell proliferation and induced apoptosis in vitro and has prolonged survival in mice with NOTCH1 mutation patient-derived T-ALL.
Targeted therapies for apoptosis
T-ALL subtypes are sensitive to BCL2 and BCL-XL inhibitors. In a study by Pullarkat et al. venetoclax plus low-dose navitoclax combination showed a positive response in 10/18 patients (ETP-ALL, n = 8; non-ETP-ALL, n = 2). Venetoclax has also shown synergistic effects with the LSD1 inhibitor GSK2879552, the MCL1-specific inhibitor S63845, the BET inhibitor JQ1, chidamide, and ruxolitinib in preclinical studies. Moreover, in a retrospective study, venetoclax plus chemotherapy achieved a CR/CR with an incomplete hematologic recovery rate of 77% in patients with R/R T-ALL/LBL. A phase I/II study is currently investigating venetoclax in combination with low-dose decitabine for patients with R/R T-ALL (NCT03808610).
A summary of clinical trials studying targeted therapies for patients with T-ALL is presented in Figure 1.
Figure 1. Ongoing clinical trials investigating targeted therapies in T-ALL*
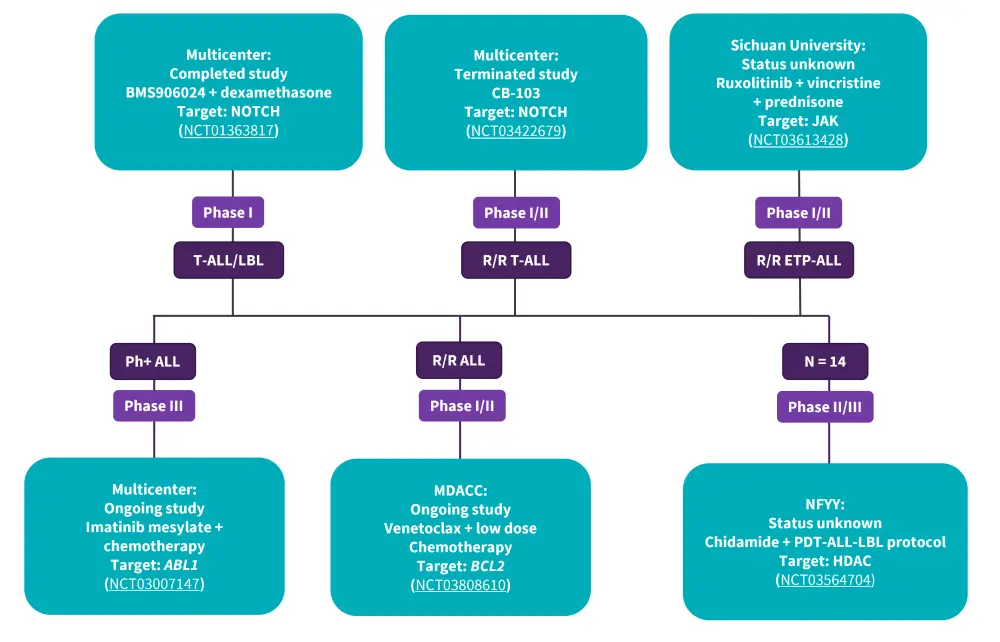
ALL, acute lymphoblastic leukemia; LBL, lymphoblastic leukemia; MDACC, MD Anderson Cancer Center; NFYY, Nanfang Hospital of Southern Medical University.
*Data from Huang, et al.1
Targeted immunotherapies
Incorporating immunotherapies is a potential strategy for improving the survival outcomes in patients with R/R T-ALL/LBL. Important targets for immunotherapies are shown in Figure 2. Key considerations for the introduction of these therapies into the clinical setting include optimizing efficacy, mitigating toxicity, overcoming the fratricide effect during chimeric antigen receptor (CAR) T-cell preparation, and selecting a safe combination with conventional therapies.
Figure 2. Key targets of Immunotherapies in T-ALL*
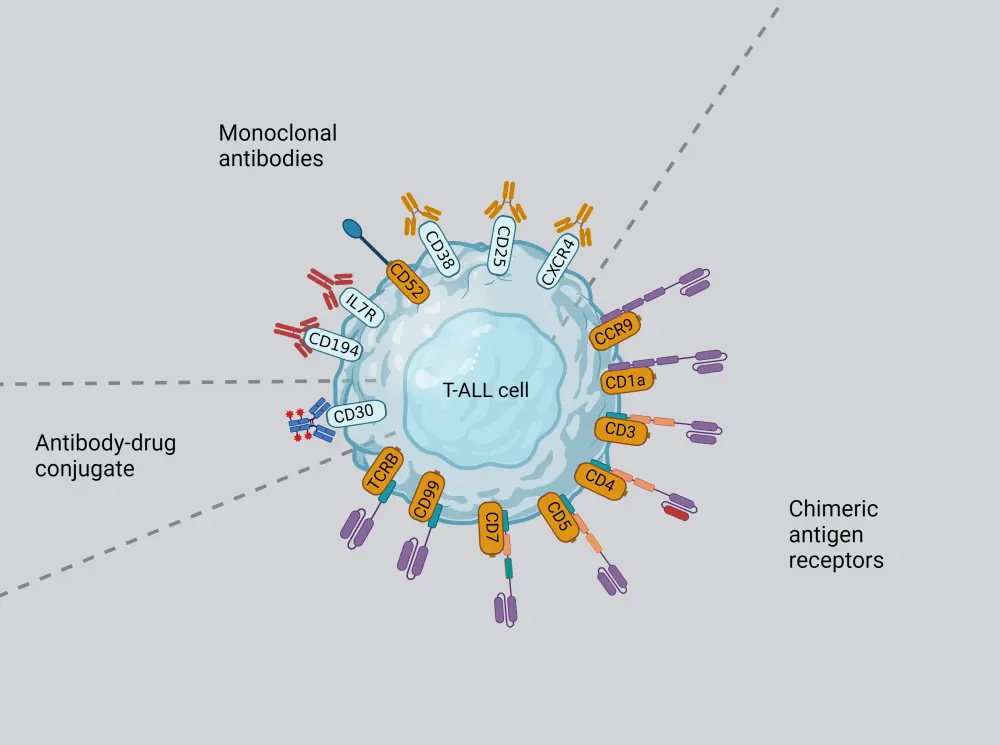
T-ALL, T-cell acute lymphoblastic leukemia.
*Data from Caracciolo, et al.2 Created with BioRender.com
Monoclonal antibodies
CD38 is widely expressed in the malignant cells of most patients with T-ALL/LBL, making anti-CD38 monoclonal antibodies an option for targeted therapy. Daratumumab, an anti-CD38 monoclonal antibody, has demonstrated promising efficacy both in preclinical models and clinical trials and is recommended by the National Comprehensive Cancer Network guidelines for the treatment of R/R T-ALL.
Isatuximab is another potential novel anti-CD38-antibody. A phase II study is currently evaluating isatuximab plus cytotoxic chemotherapy in pediatric patients with R/R T-ALL (NCT03860844); and XmAb18968, a bispecific CD3/CD38 antibody is currently being investigated for R/R T-ALL/LBL (NCT05038644).
CD7, CD1a-targeted CAR T
A study by Pan et al. which evaluated the first in-human phase I trial of donor-derived CD7 CAR T-cell therapy, reported overall response and CR rates of 95% and 85%, respectively. The median progression-free survival and overall survival was 11.0 and 18.3 months, respectively. A CR rate of 87.5% was achieved in the first study on autologous CD7 CAR T-cell treatment, with one patient with T-ALL reaching an MRD-negative CR.
Given that CD7 is widely expressed in T-ALL, it remains a promising therapeutic target for CAR T-cell therapy. However, there are several challenges with this treatment such as a shared CD7 expression on normal, malignant and CAR T-cells, causing fratricide, difficulty in obtaining a sufficient number of normal T-cells to manufacture CAR T-cells, long preparation time, and quality control of autologous CD7 CAR T-cells. Nonetheless, there are multiple clinical studies of CD7 CAR therapy currently ongoing [(NCT04984356), (NCT03690011), (NCT04033302), (NCT02742727)].
CD1a, expressed exclusively in cortical T-ALL, is detected in 33% of patients with R/R T-ALL. CD1a-specific CAR has potent cytotoxicity in vitro and in vivo, suggesting CD1a CAR T cell therapy as an attractive immunotherapy for R/R T-ALL.
CD5, CCR9, CD99-targeted CAR T
CD5 represents another suitable target for CAR T-cell therapy as it is expressed in the majority of patients with T-ALL. There are multiple clinical trials on CD5-directed CAR T-cell therapy underway [(NCT03081910), (NCT04594135), (NCT05032599)]. CD5-IL15/IL15sushi-CAR T cells were shown to decrease blasts in the cerebrospinal fluid to approximately 2% in one patient with T-ALL with central nervous system involvement, thus it could be an effective treatment option for this patient subset.
CCR9 and CD99 are highly expressed in R/R T-ALL/LBL and newly diagnosed T-ALL, respectively and have demonstrated strong anti-leukemic activity in both in vitro and in vivo experiments. There are two clinical studies on CD99 CAR T-cell therapy currently underway [(ChiCTR2100046764), (ChiCTR2000033989)].
Combination CAR T-cell and CAR-NK immunotherapy
A combination of CD3 and CD7 CAR T-cell therapy has demonstrated potent anti-leukemic activity both in vitro and in vivo, with the exception of the CD3-CD7- cohort. Additionally, a tandem CD5/CD7 bispecific CAR T-cell therapy has shown promising anti-leukemic effects.
CAR-NK treatment represents a feasible treatment for T-ALL, due to its lower risk of off-target effect, cytokine release syndrome, and neurotoxicity. However, its challenges include the optimal source of NK cells, the optimal transduction vector system, the most biologically relevant signaling domains for CAR activation, and the preservation and persistence of CAR-NK cells. Both CD7 and CD5 CAR-NK cells have demonstrated high efficacy in preclinical T-ALL studies. A clinical trial on CD5-CAR-NK (NCT05110742) is currently being investigated for R/R hematological malignancies.
Immune checkpoint inhibitors
The PD-1 molecule was identified as a marker of T-ALL by Xu et al.; they suggested the potential of conventional chemotherapy plus PD-1 blocking as a possible therapy for T-ALL. A phase II trial of monoclonal anti-PD-1 antibody pembrolizumab for R/R T-ALL has not yielded satisfactory results, thus these treatments warrant further exploration.
Conclusion
T-ALL/LBL is an aggressive malignancy with high relapse rates. The use of next-generation sequencing has provided a better understanding of genetic abnormalities at disease onset and at relapse to guide a more precise diagnosis and treatment strategy. This review highlights the progress of novel molecular targeted therapies and immunotherapies that have shown promising efficacy in preclinical and clinical settings. These novel therapies will hopefully translate into improved treatment outcomes for patients with T-ALL/LBL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


