All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Real-world outcomes of brexu-cel in R/R adult ALL
Question 1 / 2
In the multivariate analysis of the Real-World Outcomes Collaborative of CAR T in Adult ALL (ROCCA) study, which of the following factors was significantly associated with a higher progression-free survival?
A
Ages 40–59 years
B
Prior exposure to inotuzumab ozogamicin
C
Receipt of bridging therapy
D
Receipt of post brexu-cel consolidation/maintenance
Brexucabtagene autoleucel (brexu-cel) is an autologous, CD19-directed chimeric antigen receptor T-cell therapy that has been approved for the treatment of adults with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). This approval was based on results from the ZUMA-3 trial (NCT02614066), in which brexu-cel demonstrated high complete remission (CR)/CR with incomplete count recovery (CRi), and a median overall survival (OS) of 47 months in responders at the 3-year follow-up.1
In this article, we summarize four key studies on the real-world outcomes of brexu-cel in adult patients with R/R B-ALL, individually presented by Bezerra,1 Roloff,2 Rabian,3 and Kopmar4 at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition.
Real-world outcomes of brexu-cel in R/R adult B-ALL: Center for International Blood and Marrow Transplant Research registry1
Real-world outcomes of brexu-cel in adults with R/R B-ALL prospectively enrolled between 2021 and June 23, 2023 in the Center for International Blood and Marrow Transplant Research registry were presented by Bezerra.1 The outcomes of interest were CR/CRi rates, duration of response, relapse-free survival (RFS), OS, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and prolonged cytopenias.
Results
Of the 230 patients treated, 150 were included in the analysis. Patients had a diverse ethnic background and were heavily pretreated. Based on the baseline characteristics, 90% of the cohort would have been ineligible for the ZUMA-3 trial.
Efficacy
Among the efficacy evaluable patients, the overall CR/CRi rate was 79%, this rate was consistent across age, prior therapies, and the presence of extramedullary disease (EMD) (Figure 1). Among patients who were not in CR/CRi before brexu-cel infusion (n = 96), the CR/CRi rate was 66%.
Figure 1. CR/CRi rates across different subgroups*
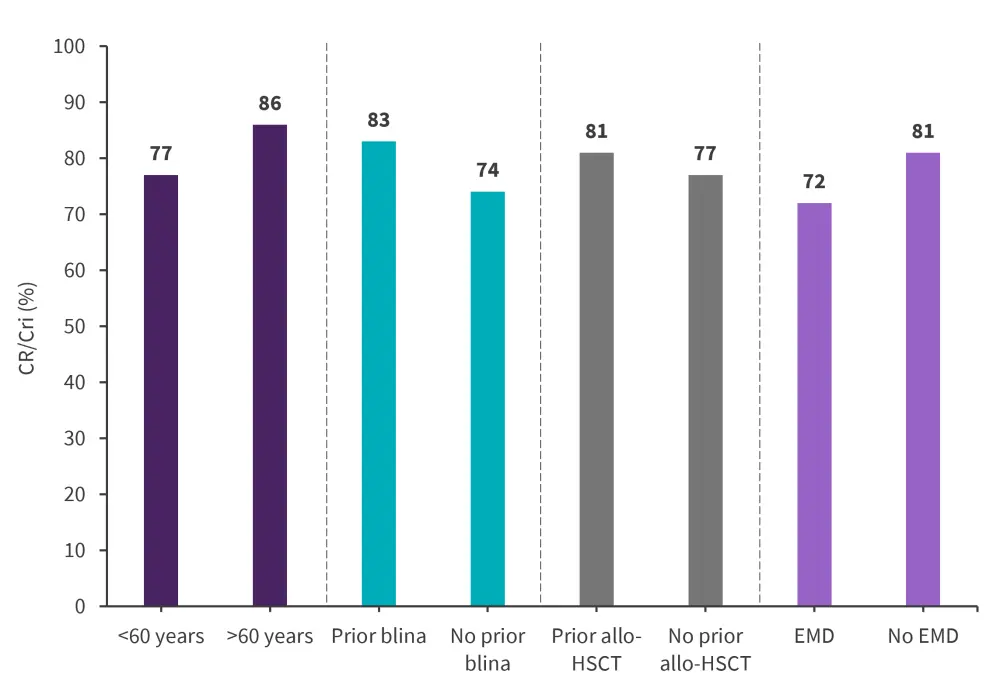
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; blina, blinatumomab; CR, complete remission; CRi, complete remission with incomplete hematological recovery; EMD, extramedullary disease.
*Adapted from Bezerra.1
At a median follow-up of 6.1 months, the 6-month duration of response, RFS, and OS was 72%, 56%, and 79%, respectively; this was consistent across prior therapy and disease status. The rate of subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT) among responders was 30%; this was consistent among patients aged <60 years, with and without prior blinatumomab, with and without EMD, with central nervous system (CNS) disease, and those not in CR/CRi after prior therapy.
Safety
Among patients in the safety set, any-grade CRS and Grade ≥3 CRS occurred in 80% and 9%, respectively. Any-grade ICANS and Grade ≥3 ICANS was seen in 47% and 23%, respectively. The median time from onset to resolution for both CRS and ICANS was 6 days, with 50% of patients requiring corticosteroids and 64% requiring tocilizumab. Prolonged cytopenias occurred in 41%, with Grade 4 thrombocytopenia and cytopenia seen in 33% and 31%, respectively. Clinically significant infections occurred in 51%, most of which were bacterial (31%) or viral infections (23%).
Presenter’s conclusion
In this prospective cohort study, brexu-cel demonstrated promising and consistent efficacy across different subgroups with a manageable safety profile consistent with clinical trial settings. Longer follow-up and larger subgroup analyses of this study are currently being planned.
Real-world outcomes of brexu-cel in R/R adult B-ALL: ROCCA2
Data from the Real-World Outcomes Collaborative of CAR T in Adult ALL (ROCCA) study was presented by Roloff.2 Study endpoints included response and survival rates, toxicities, and predictors of progression-free survival (PFS). Patients treated with brexu-cel who had a minimum of 3 months of follow-up at data cut-off were included.
Results
A total of 189 patients were included in the analysis. Patients were heavily pretreated with a median of four prior lines of therapy and over half had Philadelphia chromosome-negative disease. Based on disease burden, most patients would have been excluded from ZUMA-3.
Toxicity profile
Overall, 157 out of 187 patients experienced any-grade CRS which were mostly Grade 1–2, with only 11% of patients with Grade 3–4 CRS. 105 out of 188 patients had any-grade ICANS, 24% of which were Grade 1–2 and 31% Grade 3–4.
Response and survival
Overall, 90% of patients achieved CR/CRi, most of which were measurable residual disease (MRD)-negative CRs (Figure 2). Of the 35 patients with CNS 2/3 disease status, 12 converted to CNS 1 status without the use of intrathecal bridging therapy, this is due to brexu-cel infusion.
Figure 2. Systemic A CR/CRi rates and B MRD response*
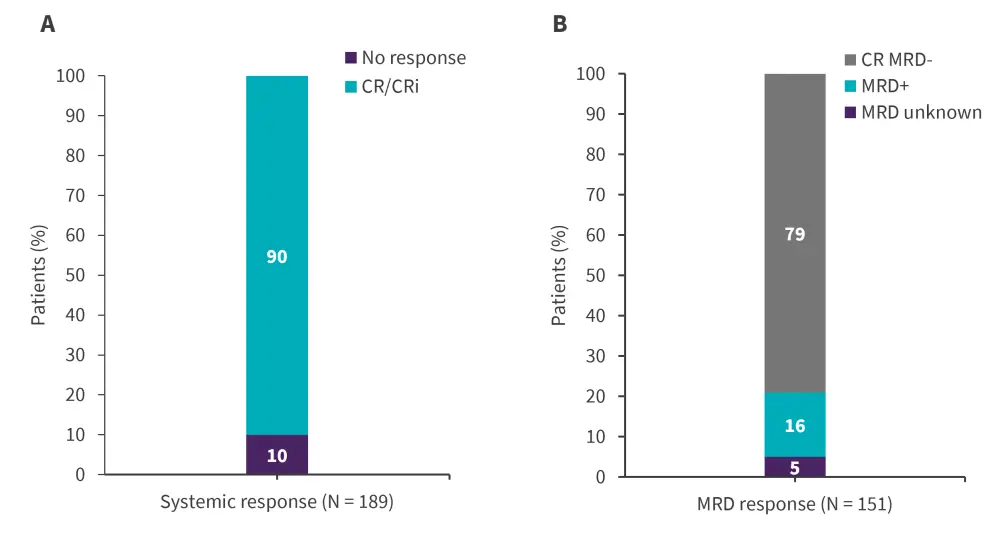
CR, complete remission; CRi, complete remission with incomplete hematological recovery; MRD, measurable residual disease.
*Adapted from Roloff.2
At a median follow-up of 11.4 months, the 6-month and 12-month PFS were 59% and 48%, respectively. With a median PFS of 9.5 months, the 6- and 12-month OS were 78% and 63%, respectively. The median OS was not reached.
In the multivariate analyses, patients aged >60 years, Asians, prior allo-HSCT, and receiving post brexu-cel consolidation/maintenance were significantly associated with a higher PFS. Conversely, prior exposure to inotuzumab ozogamicin (InO) and receipt of bridging therapy was significantly associated with a lower PFS.
In the landmark analysis (n = 157), receiving tyrosine kinase inhibitor maintenance or allo-HSCT after brexu-cel infusion was significantly associated with a higher PFS when compared with those receiving other or no maintenance. Furthermore, the use of consolidation/maintenance strategies in both MRD-negative and MRD-positive patients was significantly associated with a higher PFS.
Presenter’s conclusion
In this ROCCA study, commercial brexu-cel achieved high MRD-negative CRs and promising 1-year PFS and OS rates in adult patients with R/R B-ALL. While rates of high-grade CRS are low, Grade 3–4 ICANS was observed in 31% of patients. This study showed that ages >60 years, prior HSCT, and the use of tyrosine kinase inhibitor or HSCT as maintenance or consolidation are significant predictors of an improved PFS.
Efficacy and tolerance of brexu-cel in R/R adult B-ALL: GRAALL study from DESCAR-T registry3
Real-life data on the efficacy and safety of brexu-cel in adult patients with R/R B-ALL enrolled on the DESCAR-T registry between July 2018 and June 2023 were presented by Rabian.3 The study endpoints included best overall response, OS, and RFS, as well as the grading and incidence of ICANS and CRS.
Results
A total of 66 patients were infused with brexu-cel, and 64 had available response assessment. A third of the patients had Philadelphia chromosome-positive ALL and half of the patients were pretreated with a median of three prior lines of treatment.
Efficacy and safety
At a median follow-up of 13 months, 77% of patients achieved a CR, with 23 out of 25 patients attaining an MRD-negative CR. Older age, prior allo-HSCT, and CRS occurrence were significantly associated with higher CR rates.
In the safety cohort (n = 62), any-grade CRS was seen in 77% (6% Grade >3 CRS). Any-grade ICANS was seen in 45% (8% Grade >3 ICANS).
The median RFS was 12.9 months and the median OS was 15.6 months for all patients. For patients achieving a CR, the median OS was 25.8 months. There was no association between the number of prior lines of therapies, prior InO, prior blinatumomab, or pre-lymphodepletion bone marrow blasts percentage and outcomes; however, patients receiving prior allo-HSCT had a significantly better OS (p = 0.04) and patients with CNS involvement before leukapheresis had a significantly shorter RFS (p = 0.002) and OS (p = 0.008).
Presenter’s conclusion
In this real-world multicenter study, brexu-cel demonstrated promising efficacy and an acceptable safety profile in adult patients with R/R B-ALL. Data from this study suggest that patients with CNS involvement, who would have been ineligible for ZUMA-3, have limited benefit with brexu-cel when compared with other patients.
Real-world toxicity profile of brexu-cel in R/R adult B-ALL: ROCCA4
The toxicity profile of brexu-cel in adults with R/R B-ALL from the ROCCA multicenter study was presented by Kopmar.4 The outcomes of interest were CRS, ICANS, and other toxicities. Patients who received U.S Food and Drug Administration (FDA) approved brexu-cel between October, 2021 until June 30, 2023, across 25 centers in the US and had at least 3 months of follow-up were included in this analysis.
Results
A total of 152 patients were evaluated for response, the majority of whom received a median of four prior lines of therapy, and 51% with ≥5% blasts at apheresis.
Incidence of CRS and ICANS
Any-grade CRS occurred in 82% of patients (9% of patients experienced Grade 3+ CRS). Any-grade ICANS was seen in 56% of patients (Grade 3+ ICANS occurred in 31%). A total of 133 patients developed CRS and/or ICANS. The rates of any-grade CRS/ICANS and Grade 3+ ICANS were similar between ROCCA and ZUMA-3, but the rates of severe CRS were significantly lower in ROCCA (Figure 3).
Figure 3. Incidence of CRS and ICANS in ZUMA-3 vs ROCCA*
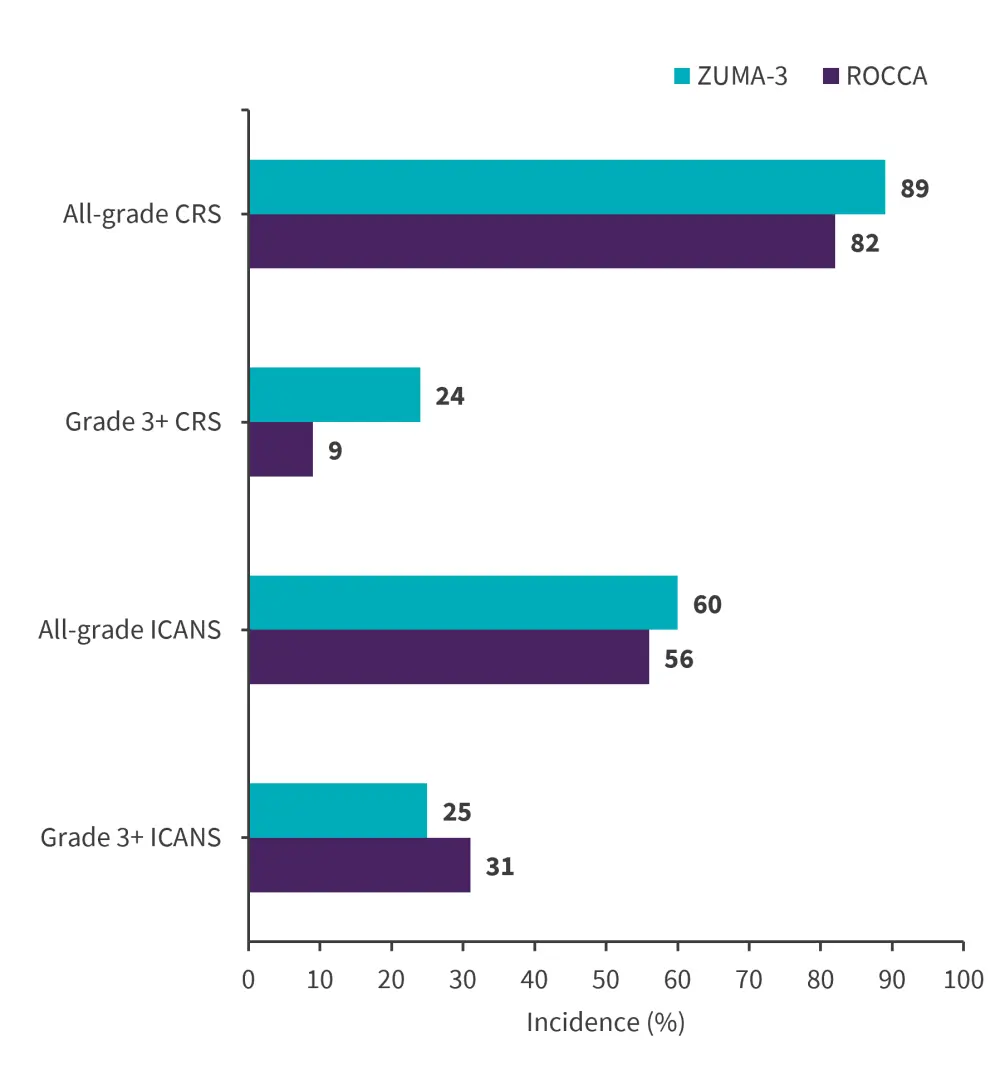
CRS, Cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ROCCA, Real-World Outcomes Collaborative of CAR T in Adult ALL.
*Adapted from Kopmar.4
Overall, 68% of patients received tocilizumab for the treatment of CRS/ICANS; 31% of these patients responded and 9% were tocilizumab-refractory. Of the 61% of patients who received corticosteroids, 37% responded to the first steroid trial and 10% were steroid refractory. 21% of the study cohort received anakinra and 4% of patients received other agents for the management of CRS/ICANS.
Univariate analyses revealed a significant association between active disease (≥5% marrow blasts and/or EMD) and Grade 3+ ICANS (p = 0.008). The Cox aggression analysis showed a significant association between Grade 3+ CRS and death (p = 0.05).
Incidence of other toxicities
Hemophagocytic lymphohistiocytosis was reported in 6 patients which overlapped with infections or occurred after CRS/ICANS. The most common infections that occurred between Day 0 and Day 28 were bacterial (n = 12), fungal (n = 6), pneumonia (n = 3), and cytomegalovirus (n = 4). A total of 9 deaths occurred by Day 28 of the study due to CRS (n = 3), ICANS (n = 3), infections (n = 5), disease progression (n = 2), and hemophagocytic lymphohistiocytosis (n = 1).
Presenter’s conclusion
This real-world study reported similar rates of severe ICANS, but a lower incidence of severe CRS compared to ZUMA-3. Severe ICANS was significantly associated with active disease, and severe CRS was associated with a higher risk of death.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


