All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Two-year follow-up of KTE-X19 in adult patients with R/R B-ALL: ZUMA-3 trial
The overall prognosis in adult patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) is dismal, with reported median overall survival (OS) of <8 months with standard therapies. Brexucabtagene autoleucel (KTE-X19) became the first chimeric antigen receptor (CAR) T-cell therapy to be approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with acute lymphoblastic leukemia (ALL) in 2021. KTE-X19 has also been approved in the US to treat adults with R/R B-ALL and adults with R/R mantle cell lymphoma based on the results of the ZUMA-3 trial (NCT02614066). The ALL Hub previously reported an interview with Bijal Shah on the safety and efficacy of KTE-X19 in adult patients with R/R B-ALL.
KTE-X19 showed significant efficacy and a tolerable safety profile in heavily pretreated adults with R/R B-ALL during phase II of the ZUMA-3 trial. During the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Bijal Shah presented the updated outcomes with 2-year follow-up in treated phase II patients, and a larger pooled analysis of treated phase I and II patients who received a dose of KTE-X19 in ZUMA-3 trial. Below, we summarize the key findings.
Study design
This is an ongoing, phase II part of the ZUMA-3 trial in patients aged ≥18 years with R/R B-ALL and bone marrow (BM) blasts >5%, who may have received prior blinatumomab and/or prior allogenic hematopoietic stem cell transplantation (allo-HSCT). All patients received colony-stimulating prophylaxis of an intrathecal regimen along with bridging therapy, particularly for those with >25% BM blasts or >1000 blasts/µL of peripheral blood at screening. Patients received conditioning chemotherapy followed by the pivotal dose of KTE-X19 (Figure 1).
Figure 1. Study design*
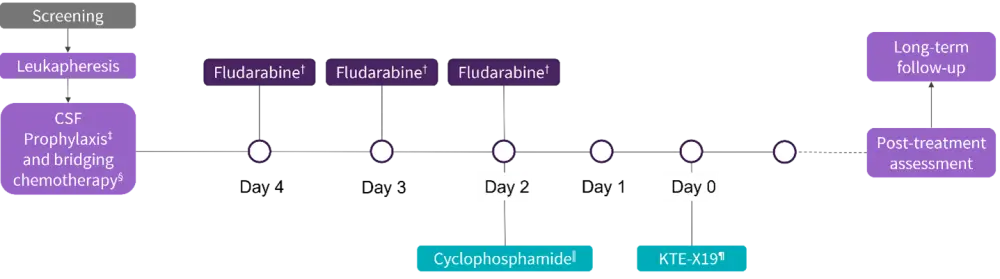
CSF, colony stimulating factor; KTE-X19, brexucabtagene autoleucel.
*Adapted from Shah.1
†25 mg/m2 intravenous.
‡All patients received CSF prophylaxis consisting of an intrathecal regimen according to institutional or national guidelines.
§recommended for all patients particularly those with >25% marrow blasts or >1000 blasts/µL of peripheral blood at screening.
‖900 mg/m2 intravenous.
¶1 × 106 anti-CD19 CAR T-cells/kg.
The primary endpoint was the overall complete remission (CR) rate (CR + complete remission with incomplete hematologic recovery [CRi]). Secondary endpoints included duration of response, relapse-free survival, subsequent allo-HSCT, overall survival (OS), safety, and CAR T-cell levels in the blood.
Post hoc subgroup analysis was performed based on:
- Age (18–25, 18–39, 40–59, ≥60 years).
- Baseline BM blasts percentage (0–5%, > 5–25%, > 25–50%, > 50–75%, >75–100%) following bridging therapy and prior to KTE-X19 infusion.
Results
Baseline characteristics
A total of 55 and 78 patients treated in phase II and pooled phase I and II, respectively, were included. The baseline characteristics were largely similar among the subgroups by age or baseline BM blasts. The median follow-up was 26.8 months (range, 20.7–32.6 months) for treated phase II patients and 29.7 (range, 20.7–58.3 months) for pooled phase I and II treated patients with a median number of two prior therapies.
Response rates and OS
At the data cut-off of July 2021, the median duration of remission was ≥20 months in treated phase II patients and the CR/CRi rate was 71%, with 56% and 15% of patients achieving CR and CRi, respectively. Of the 39 patients who achieved CR/CRi, 26% of patients received subsequent allo-HSCT and 36% relapsed (Figure 2). The subsequent allo-HSCT rate following KTE-X19 was 20%. Median duration of response and OS in patients with subsequent allo-HSCT was not reached.
Figure 2. Consort diagram of treated phase II patients*
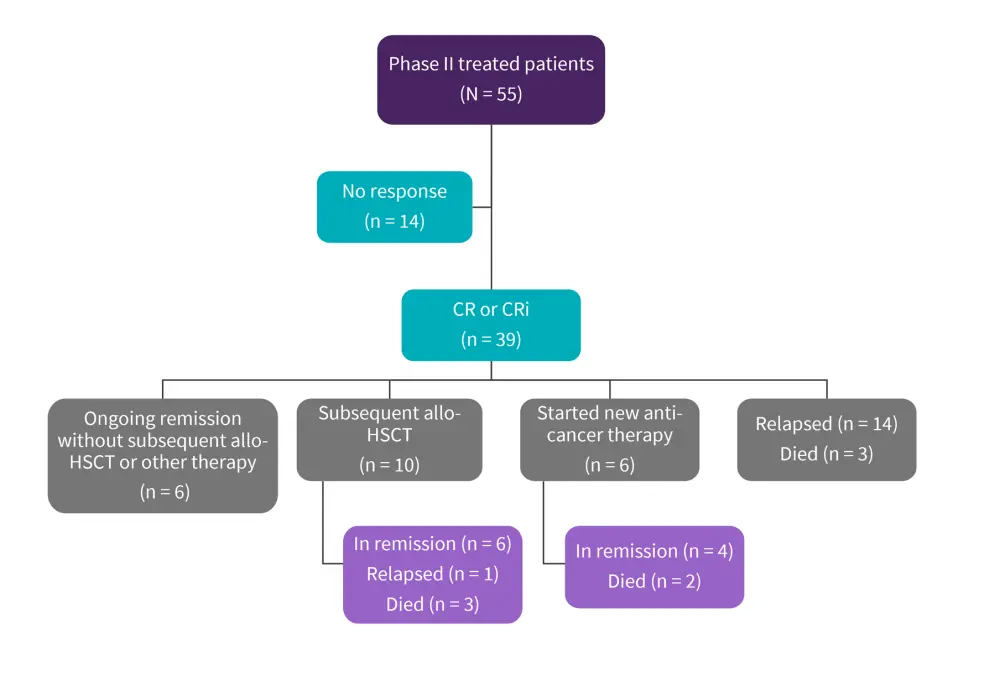
Allo-HSCT, allogenic hematopoietic stem cell transplantation; CR, complete remission; CRi, complete remission with incomplete hematologic recovery.
*Adapted from Shah.1
Subgroup analysis
Response rates among the prespecified subgroups were consistent with the all-treated population however, lower response rate was observed in patients with >75% baseline BM blasts (Table 1).
Table 1. Efficacy and durability of outcomes*
|
Allo-HSCT, allogenic hematopoietic stem cell transplantation; BFBM, blast-free hypoplastic or aplastic bone marrow; BM, bone marrow; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; DOR, duration of response; NE, not estimable; NR, not reached; OS, overall survival; RFS, relapse-free survival. ‡Assessed by independent central review. |
||||||||
|
Outcome, % |
ORR†,‡ |
CR‡ |
CRi‡ |
BFBM‡ |
No response‡ |
Median DOR§ |
Median RFS§ |
Median OS |
|---|---|---|---|---|---|---|---|---|
|
Phase II treated |
70.9 |
56.4 |
14.5 |
7.3 |
16.4 |
14.6 (9.4–NE) |
11.6 (2.7–20.5) |
25.4 (16.2–NE) |
|
Age, years |
||||||||
|
18–25 |
66.7 |
58.3 |
8.3 |
8.3 |
8.3 |
16.6 (14.6–NE) |
15.5 (0.0–NE) |
NR (0.6–NE) |
|
18–39 |
61.5 |
53.8 |
7.7 |
7.7 |
19.2 |
18.6 (12.8–NE) |
14.2 (0.0–20.5 |
25.4 (9.5–NE) |
|
40–59 |
70.0 |
55.0 |
15.0 |
10.0 |
20.0 |
10.3 (1.3–NE) |
11.6 (0.0–NE) |
26.0 (7.6–NE) |
|
≥60 |
100 |
66.7 |
33.0 |
0.0 |
0.0 |
9.4 (1.8–NE) |
11.7 (2.8–NE) |
NR (12.2–NE) |
|
Baseline BM blasts, % |
||||||||
|
≤5 |
80.0 |
80.0 |
0 |
20.0 |
0.0 |
14.4 (1.3–NE) |
5.6 (0.0–NE) |
NR (8.8–NE) |
|
>5–25 |
90.0 |
70.0 |
20.0 |
0.0 |
10.0 |
23.6 (1.8–NE) |
25.4 (0.0–NE) |
25.4 (8.3–NE) |
|
>25–50 |
90.9 |
81.8 |
9.1 |
0.0 |
0.0 |
18.6 (9.4–NE) |
20.5 (10.3–NE) |
NR (9.0–NE) |
|
>50–75 |
80.0 |
50.0 |
30.0 |
10.0 |
10.0 |
20.0 (1.0–NE) |
6.1 (0.0–NE) |
NR (2.1–NE) |
|
>75–100 |
42.1 |
31.6 |
10.5 |
10.5 |
36.8 |
9.6 (0.8–12.8) |
0.0 (0.0–11.6) |
14.2 (2.2–NE) |
|
Phase I and II‖ (n = 78) |
73.1 |
60.3 |
12.8 |
7.7 |
15.4 |
18.6 (9.6–NE) |
11.7 (6.1–20.5) |
25.4 (16.2–NE) |
|
Age, years |
||||||||
|
18–25 |
73.3 |
60.0 |
13.3 |
6.7 |
6.7 |
14.6 (0.7–NE) |
15.5 (0.0–NE) |
23.2 (9.0–NE) |
|
18–39 |
69.4 |
58.3 |
11.1 |
5.6 |
16.7 |
18.6 (12.8–NE) |
14.2 (2.3–NE) |
23.2 (14.2–NE) |
|
40–59 |
70.4 |
59.3 |
11.1 |
11.1 |
18.5 |
20.0 (4.7–NE) |
7.7 (0.0–22.1) |
26.0 (9.3–NE) |
|
≥60 |
86.7 |
66.7 |
20.0 |
6.7 |
6.7 |
NR (1.8–NE) |
14.4 (2.8–NE) |
47.0 (12.2–NE) |
|
Baseline BM blasts, % |
||||||||
|
≤5 |
75.0 |
75.0 |
0.0 |
12.5 |
12.5 |
4.9 (1.3–NE) |
5.6 (0.0–NE) |
26.0 (2.2–NE) |
|
>5–25 |
85.7 |
71.4 |
14.3 |
7.1 |
7.1 |
23.6 (1.8–NE) |
25.4 (2.8–NE) |
25.4 (21.9–NE) |
|
>25–50 |
83.3 |
75.0 |
8.3 |
8.3 |
0.0 |
18.6 (9.4–NE) |
20.5 (0.0–NE) |
NR (1.7–NE) |
|
>50–75 |
85.7 |
57.1 |
28.6 |
7.1 |
7.1 |
20.0 (5.2–NE) |
22.1 (1.8–NE) |
NR (9.5–NE) |
|
>75–100 |
56.7 |
46.7 |
10.0 |
6.7 |
30.0 |
10.3 (1.3–NE) |
2.7 (0.0–11.7) |
16.1 (9.5–NE) |
OS
The median OS was 25.4 months for both cohorts and was not reached for treated phase II patients with CR. Similarly, median OS was not reached in patients aged 18–25 or ≥60 years or in those with ≤5%, >25–50%, or >50–75% baseline BM blasts. Estimated 24-month OS was similar among the prespecified subgroups, with the lowest rates observed in patients with >75% blasts (OS rate = 29%).
With regards to blood CAR T-cell levels after KTE-X19, the median time to peak was 15 days in treated phase II patients. CAR T-cell levels were undetectable in 79% (22/28) of evaluable patients by Month 6 and in all evaluable patients (n = 10), including ongoing responders, by Month 24.
Safety
There was no occurrence of any new-onset adverse events (AEs) such as cytokine release syndrome, neurological event, infections, or hypogammaglobulinemia of any grade since the primary analysis of phase II, with the exception of one new Grade 5 AE (graft-versus-host disease). In total, four patients have died since the primary analysis, one due to progressive disease and three due to unrelated causes. Similar incidences of Grade ≥3 cytokine release syndrome and neurological events between age and BM blasts subgroups were observed (Table 2).
Table 2. Adverse events by subgroups*
|
BM, bone marrow; CRS, cytokine release syndrome. |
||||||
|
|
CRS, % |
Neurological events, % |
||||
|---|---|---|---|---|---|---|
|
|
Grade 1 |
Grade 2 |
Grade ≥3 |
Grade 1 |
Grade 2 |
Grade ≥3 |
|
Age, years |
||||||
|
18–25 (n = 12) |
25.0 |
50.0 |
25.0 |
0.0 |
25.0 |
42.0 |
|
18–39 (n = 26) |
23.0 |
46.0 |
23.0 |
12.0 |
15.0 |
31.0 |
|
40–59 (n = 20) |
15.0 |
45.0 |
20.0 |
10.0 |
35.0 |
20.0 |
|
≥60 (n = 9) |
22.0 |
44.0 |
33.0 |
11.0 |
22.0 |
22.0 |
|
Baseline BM blasts, % |
||||||
|
≤5 (n = 5) |
20.0 |
40.0 |
20.0 |
20.0 |
40.0 |
20.0 |
|
>5–25 (n = 10) |
20.0 |
60.0 |
10.0 |
0.0 |
10.0 |
30.0 |
|
>25–50 (n = 11) |
18.2 |
54.5 |
27.3 |
0.0 |
27.3 |
54.5 |
|
>50–75 (n = 10) |
20.0 |
50.0 |
10.0 |
20.0 |
20.0 |
10.0 |
|
>75–100 (n = 19) |
21.1 |
31.6 |
36.8 |
15.8 |
26.3 |
15.8 |
Conclusion
The updated findings from the ZUMA-3 trial demonstrate that with longer follow-up and a larger pooled data set, outcomes remained stable over time in adult patients with R/R B-ALL. KTE-X19 benefited most patients, irrespective of their age and baseline BM blast percentage, but provided less benefit to those with >75% BM blasts. The safety profile showed the treatment was well tolerated, with no new AEs since the primary analysis of phase II. The pharmacokinetic profile suggested that the persistence of CAR T-cells in the blood is not required for durable responses. Overall, the outcomes among treated phase II patients and the pooled analysis of phase I and II were similar; future ongoing trials will indicate if these results remain consistent with a larger patient cohort in a real-world setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

